Phorbol-12-myristate-13-acetate is a potent enhancer of B cells with a granzyme B+ regulatory phenotype
- PMID: 37588597
- PMCID: PMC10426744
- DOI: 10.3389/fimmu.2023.1194880
Phorbol-12-myristate-13-acetate is a potent enhancer of B cells with a granzyme B+ regulatory phenotype
Abstract
Introduction: The infusion of ex-vivo-generated regulatory B cells may represent a promising novel therapeutic approach for a variety of autoimmune and hyperinflammatory conditions including graft-versus-host disease.
Methods: Previously, we developed a protocol for the generation of a novel population of regulatory B cells, which are characterized by secretion of enzymatically active granzyme B (GraB cells). This protocol uses recombinant interleukin 21 (IL-21) and goat-derived F(ab)'2 fragments against the human B cell receptor (anti-BCR). Generally, the use of xenogeneic material for the manufacturing of advanced therapy medicinal products should be avoided to prevent adverse immune reactions as well as potential transmission of so far unknown diseases.
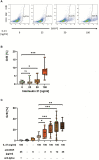
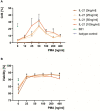
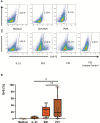
Results: In the present work we demonstrated that phorbol-12-myristate-13-acetate (PMA/TPA), a phorbol ester with a particular analogy to the second messenger diacylglycerol (DAG), is a potent enhancer of IL-21-induced differentiation of pre-activated B cells into GraB cells. The percentage of GraB cells after stimulation of pre-activated B cells with IL-21 and PMA/TPA was not significantly lower compared to stimulation with IL-21 and anti-BCR.
Discussion: Given that PMA/TPA has already undergone encouraging clinical testing in patients with certain haematological diseases, our results suggest that PMA/TPA may be a safe and feasible alternative for ex-vivo manufacturing of GraB cells.
Keywords: 12-O-tetradecanoylphorbol-13-acetate; GraB cells; GvHD; PMA; TPA; granzyme B; regulatory B cells.
Copyright © 2023 Veh, Mangold, Felsen, Ludwig, Gerstner, Reinhardt, Schrezenmeier, Fabricius and Jahrsdörfer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, et al. . Distinct effector B cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity (2018) 49:725–39.e726. doi: 10.1016/j.immuni.2018.08.015 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

