Dual pH and microbial-sensitive galactosylated polymeric nanocargoes for multi-level targeting to combat ulcerative colitis
- PMID: 37588990
- PMCID: PMC10425895
- DOI: 10.1016/j.ajps.2023.100831
Dual pH and microbial-sensitive galactosylated polymeric nanocargoes for multi-level targeting to combat ulcerative colitis
Abstract
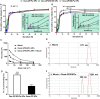
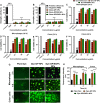
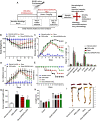
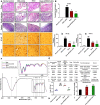
Ulcerative colitis (UC) is a type of inflammatory bowel disease characterized by inflammation, ulcers and irritation of the mucosal lining. Oral drug delivery in UC encounters challenges because of multifaceted barriers. Dexamethasone-loaded galactosylated-PLGA/Eudragit S100/pullulan nanocargoes (Dexa-GP/ES/Pu NCs) have been developed with a dual stimuli-sensitive coating responsive to both colonic pH and microbiota, and an underneath galactosylated-PLGA core (GP). The galactose ligand of the GP preferentially binds to the macrophage galactose type-lectin-C (MGL-2) surface receptor. Therefore, both stimuli and ligand-mediated targeting facilitate nanocargoes to deliver Dexa specifically to the colon with enhanced macrophage uptake. Modified emulsion method coupled with a solvent evaporation coating technique was employed to prepare Dexa-GP/ES/Pu NCs. The nanocargoes were tested using in vitro, ex vivo techniques and dextran sodium sulfate (DSS) induced UC model. Prepared nanocargoes had desired physicochemical properties, drug release, cell uptake and cellular viability. Investigations using a DSS-colitis model showed high localization and mitigation of colitis with downregulation of NF-ĸB and COX-2, and restoration of clinical, histopathological, biochemical indices, antioxidant balance, microbial alterations, FTIR spectra, and epithelial junctions' integrity. Thus, Dexa-GP/ES/Pu NCs found to be biocompatible nanocargoes capable of delivering drugs to the inflamed colon with unique targeting properties for prolonged duration.
Keywords: Galactosylated nanocargoes; Macrophage galactose type-lectin C; Microbial sensitive; Pullulan; Ulcerative colitis; pH-sensitive drug delivery.
© 2023 Shenyang Pharmaceutical University.
Conflict of interest statement
The authors declared no conflict of interest.
Figures




References
-
- Tatiya-aphiradee N., Chatuphonprasert W., Jarukamjorn K. Immune response and inflammatory pathway of ulcerative colitis. J Basic Clin Physiol Pharmacol. 2019;30(1):1–10. - PubMed
-
- Mukhtar M., Ali H., Ahmed N., Munir R., Talib S., Khan A.S., et al. Drug delivery to macrophages: a review of nano-therapeutics targeted approach for inflammatory disorders and cancer. Expert Opin Drug Deliv. 2020;17(9):1239–1257. - PubMed
-
- Zeeshan M., Ali H., Khan S., Khan S.A., Weigmann B. Advances in orally-delivered pH-sensitive nanocarrier systems; an optimistic approach for the treatment of inflammatory bowel disease. Int J Pharm. 2019;558:201–214. - PubMed
-
- Xu Y., Zhu B.W., Li X., Li Y.F., Ye X.M., Hu J.N. Glycogen-based pH and redox sensitive nanoparticles with ginsenoside Rh2 for effective treatment of ulcerative colitis. Biomaterials. 2021 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

