Cardiac Genetic Investigation of Sudden Infant and Early Childhood Death: A Study From Victims to Families
- PMID: 37589201
- PMCID: PMC10547337
- DOI: 10.1161/JAHA.122.029100
Cardiac Genetic Investigation of Sudden Infant and Early Childhood Death: A Study From Victims to Families
Abstract
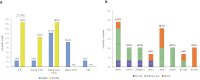
Background Sudden infant death syndrome (SIDS) is the leading cause of death up to age 1. Sudden unexplained death in childhood (SUDC) is similar but affects mostly toddlers aged 1 to 4. SUDC is rarer than SIDS, and although cardiogenetic testing (molecular autopsy) identifies an underlying cause in a fraction of SIDS, less is known about SUDC. Methods and Results Seventy-seven SIDS and 16 SUDC cases underwent molecular autopsy with 25 definitive-evidence arrhythmia-associated genes. In 18 cases, another 76 genes with varying degrees of evidence were analyzed. Parents were offered cascade screening. Double-blind review of clinical-genetic data established genotype-phenotype correlations. The yield of likely pathogenic variants in the 25 genes was higher in SUDC than in SIDS (18.8% [3/16] versus 2.6% [2/77], respectively; P=0.03), whereas novel/ultra-rare variants of uncertain significance were comparably represented. Rare variants of uncertain significance and likely benign variants were found only in SIDS. In cases with expanded analyses, likely pathogenic/likely benign variants stemmed only from definitive-evidence genes, whereas all other genes contributed only variants of uncertain significance. Among 24 parents screened, variant status and phenotype largely agreed, and 3 cases positively correlated for cardiac channelopathies. Genotype-phenotype correlations significantly aided variant adjudication. Conclusions Genetic yield is higher in SUDC than in SIDS although, in both, it is contributed only by definitive-evidence genes. SIDS/SUDC cascade family screening facilitates establishment or dismissal of a diagnosis through definitive variant adjudication indicating that anonymity is no longer justifiable. Channelopathies may underlie a relevant fraction of SUDC. Binary classifications of genetic causality (pathogenic versus benign) could not always be adequate.
Keywords: channelopathies; molecular autopsy; sudden infant death syndrome; sudden unexplained death in childhood.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

