L. rhamnosus CNCM I-3690 survival, adaptation, and small bowel microbiome impact in human
- PMID: 37589280
- PMCID: PMC10438856
- DOI: 10.1080/19490976.2023.2244720
L. rhamnosus CNCM I-3690 survival, adaptation, and small bowel microbiome impact in human
Abstract
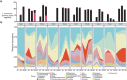
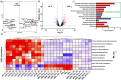
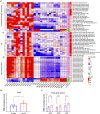
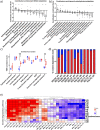
Fermented foods and beverages are a significant source of dietary bacteria that enter the gastrointestinal (GI) tract. However, little is known about how these microbes survive and adapt to the small intestinal environment. Colony-forming units (CFU) enumeration and viability qPCR of Lacticaseibacillus rhamnosus CNCM I-3690 in the ileal effluent of 10 ileostomy subjects during 12-h post consumption of a dairy product fermented with this strain demonstrated the high level of survival of this strain during human small intestine passage. Metatranscriptome analyses revealed the in situ transcriptome of L. rhamnosus in the small intestine, which was contrasted with transcriptome data obtained from in vitro cultivation. These comparative analyses revealed substantial metabolic adaptations of L. rhamnosus during small intestine transit, including adjustments of carbohydrate metabolism, surface-protein expression, and translation machinery. The prominent presence of L. rhamnosus in the effluent samples did not elicit an appreciable effect on the composition of the endogenous small intestine microbiome, but significantly altered the ecosystem's overall activity profile, particularly of pathways associated with carbohydrate metabolism. Strikingly, two of the previously recognized gut-brain metabolic modules expressed in situ by L. rhamnosus (inositol degradation and glutamate synthesis II) are among the most dominantly enriched activities in the ecosystem's activity profile. This study establishes the survival capacity of L. rhamnosus in the human small intestine and highlights its functional adjustment in situ, which we postulate to play a role in the probiotic effects associated with this strain.
Keywords: L. rhamnosus; human; in situ gene expression; metatranscriptome; probiotic fate; small intestine.
Conflict of interest statement
This author discloses the following: T. Smokvina and C. Chervaux are employees of Danone Nutricia Research. The remaining authors declare that they have no competing interests.
Figures

References
-
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–21. doi: 10.1038/nrgastro.2014.66. - DOI - PubMed
-
- Kant R, Rintahaka J, Yu X, Sigvart-Mattila P, Paulin L, Mecklin JP, Saarela M, Palva A, von Ossowski I.. A comparative pan-genome perspective of niche-adaptable cell-surface protein phenotypes in Lactobacillus rhamnosus. PLoS One. 2014;9(7):e102762. doi: 10.1371/journal.pone.0102762. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
