An NHC-Catalyzed Desulfonylative Smiles Rearrangement of Pyrrole and Indole Carboxaldehydes
- PMID: 37589318
- PMCID: PMC10476196
- DOI: 10.1021/acs.joc.3c01089
An NHC-Catalyzed Desulfonylative Smiles Rearrangement of Pyrrole and Indole Carboxaldehydes
Abstract
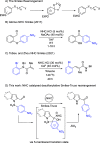
The use of catalysis methods to enable Smiles rearrangement opens up new substrate classes for arylation under mild conditions. Here, we describe an N-heterocyclic carbene (NHC) catalysis system that accesses indole and pyrrole aldehyde substrates in a desulfonylative Smiles process. The reaction proceeds under mild, transition-metal-free conditions and captures acyl anion reactivity for the synthesis of a diverse array of 2-aroyl indoles and pyrroles from readily available sulfonamide starting materials.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Wales S. M.; Saunthwal R. K.; Clayden J. C(Sp3)-Arylation by Conformationally Accelerated Intramolecular Nucleophilic Aromatic Substitution (SNAr). Acc. Chem. Res. 2022, 55, 1731–1747. 10.1021/acs.accounts.2c00184. - DOI - PMC - PubMed
- Huynh M.; De Abreu M.; Belmont P.; Brachet E. Spotlight on Photoinduced Aryl Migration Reactions. Chem.—Eur. J. 2021, 27, 3581–3607. 10.1002/chem.202003507. - DOI - PubMed
- Whalley D. M.; Greaney M. F. Recent Advances in The Smiles Rearrangement: New Opportunities for Arylation. Synthesis 2022, 54, 1908–1918. 10.1055/a-1710-6289. - DOI
-
-
Selected recent examples:
- Saunthwal R. K.; Schwarz M.; Mallick R. K.; Terry-Wright W.; Clayden J. Enantioselective Intramolecular A-Arylation of Benzylamine Derivatives: Synthesis of a Precursor to Levocetirizine. Angew. Chem., Int. Ed. 2023, 62, e20221675810.1002/anie.202216758. - DOI - PubMed
- Zhen G.; Zeng G.; Jiang K.; Wang F.; Cao X.; Yin B. Visible-Light-Induced Diradical-Mediated Ipso-Cyclization towards Double Dearomative [2 + 2]-Cycloaddition or Smiles-Type Rearrangement. Chem.—Eur. J. 2023, 29, e20220321710.1002/chem.202203217. - DOI - PubMed
- Lemmerer M.; Zhang H.; Fernandes A. J.; Fischer T.; Mießkes M.; Xiao Y.; Maulide N. Synthesis of α-Aryl Acrylamides via Lewis-Base-Mediated Aryl/Hydrogen Exchange. Angew. Chem. Int. Ed 2022, 61, e20220747510.1002/anie.202207475. - DOI - PMC - PubMed
- Allen A. R.; Poon J.-F.; McAtee R. C.; Watson N. B.; Pratt D. A.; Stephenson C. R. J. Mechanism of Visible Light-Mediated Alkene Aminoarylation with Arylsulfonylacetamides. ACS Catal. 2022, 12, 8511–8526. 10.1021/acscatal.2c02577. - DOI - PMC - PubMed
- Huang J.; Liu F.; Zeng L.-H.; Li S.; Chen Z.; Wu J. Accessing Chiral Sulfones Bearing Quaternary Carbon Stereocenters via Photoinduced Radical Sulfur Dioxide Insertion and Truce–Smiles Rearrangement. Nat. Commun. 2022, 13, 7081.10.1038/s41467-022-34836-y. - DOI - PMC - PubMed
- Chen X.; Wang Q.; Zhang Z.; Niu Z.-J.; Shi W.-Y.; Gong X.-P.; Jiao R.-Q.; Gao M.-H.; Liu X.-Y.; Liang Y.-M. Copper-Catalyzed Hydrogen Atom Transfer and Aryl Migration Strategy for the Arylalkylation of Activated Alkenes. Org. Lett. 2022, 24, 4338–4343. 10.1021/acs.orglett.2c01427. - DOI - PubMed
- Noten E. A.; McAtee R. C.; Stephenson C. R. J. Catalytic Intramolecular Aminoarylation of Unactivated Alkenes with Aryl Sulfonamides. Chem. Sci. 2022, 13, 6942–6949. 10.1039/D2SC01228F. - DOI - PMC - PubMed
- Radhoff N.; Studer A. 1,4-Aryl Migration in Ketene-Derived Enolates by a Polar-Radical-Crossover Cascade. Nat. Commun. 2022, 13, 3083.10.1038/s41467-022-30817-3. - DOI - PMC - PubMed
- Shi Z.; Li Y.; Li N.; Wang W.; Lu H.; Yan H.; Yuan Y.; Zhu J.; Ye K. Electrochemical Migratory Cyclization of N-Acylsulfonamides. Angew. Chem., Int. Ed. 2022, 61, e20220605810.1002/anie.202206058. - DOI - PubMed
- Horst B.; Verdoorn D. S.; Hennig S.; van der Heijden G.; Ruijter E. Enantioselective Total Synthesis of (−)-Limaspermidine and (−)-Kopsinine by a Nitroaryl Transfer Cascade Strategy. Angew. Chem., Int. Ed. 2022, 61, e20221059210.1002/anie.202210592. - DOI - PMC - PubMed
- Abe M.; Nitta S.; Miura E.; Kimachi T.; Inamoto K. Nitrile Synthesis via Desulfonylative–Smiles Rearrangement. J. Org. Chem. 2022, 87, 4460–4467. 10.1021/acs.joc.1c03011. - DOI - PubMed
- Cheibas C.; Fincias N.; Casaretto N.; Garrec J.; El Kaïm L. Passerini–Smiles Reaction of A-Ketophosphonates: Platform for Phospha-Brook/Smiles Embedded Cascades. Angew. Chem., Int. Ed. 2022, 61, e20211624910.1002/anie.202116249. - DOI - PubMed
- Sephton T.; Large J. M.; Butterworth S.; Greaney M. F. Diarylamine Synthesis via Desulfinylative Smiles Rearrangement. Org. Lett. 2022, 24, 1132–1135. 10.1021/acs.orglett.1c04122. - DOI - PMC - PubMed
- Hervieu C.; Kirillova M. S.; Suárez T.; Müller M.; Merino E.; Nevado C. Asymmetric, visible light-mediated radical sulfinyl-Smiles rearrangement to access all-carbon quaternary stereocentres. Nat. Chem. 2021, 13, 327–334. 10.1038/s41557-021-00668-4. - DOI - PubMed
- Kang L.; Wang F.; Zhang J.; Yang H.; Xia C.; Qian J.; Jiang G. High Chemo-/Stereoselectivity for Synthesis of Polysubstituted Monofluorinated Pyrimidyl Enol Ether Derivatives. Org. Lett. 2021, 23, 1669–1674. 10.1021/acs.orglett.1c00092. - DOI - PubMed
- Chen F.; Shao Y.; Li M.; Yang C.; Su S. J.; Jiang H.; Ke Z.; Zeng W. Photocatalyzed cycloaromatization of vinylsilanes with arylsulfonylazides. Nat. Commun. 2021, 12, 3304.10.1038/s41467-021-23326-2. - DOI - PMC - PubMed
- Radhoff N.; Studer A. Functionalization of α-C(sp3)–H Bonds in Amides Using Radical Translocating Arylating Group. Angew. Chem., Int. Ed. 2021, 60, 3561–3565. 10.1002/anie.202013275. - DOI - PMC - PubMed
- Gillaizeau-Simonian N.; Barde E.; Guérinot A.; Cossy J. Cobalt-Catalyzed 1,4-Aryl Migration/Desulfonylation Cascade: Synthesis of α-Aryl Amides. Chem.—Eur. J. 2021, 27, 4004–4008. 10.1002/chem.202005129. - DOI - PubMed
- Wang Z. S.; Chen Y. B.; Zhang H. W.; Sun Z.; Zhu C.; Ye L. W. Ynamide Smiles Rearrangement Triggered by Visible-Light-Mediated Regioselective Ketyl–Ynamide Coupling: Rapid Access to Functionalized Indoles and Isoquinolines. J. Am. Chem. Soc. 2020, 142, 3636–3644. 10.1021/jacs.9b13975. - DOI - PubMed
- Yan J.; Cheo H. W.; Teo W. K.; Shi X.; Wu H.; Idres S. B.; Deng L. W.; Wu J. A Radical Smiles Rearrangement Promoted by Neutral Eosin Y as a Direct Hydrogen Atom Transfer Photocatalyst. J. Am. Chem. Soc. 2020, 142, 11357–11362. 10.1021/jacs.0c02052. - DOI - PubMed
- Ruzi R.; Ma J.; Yuan X.; Wang W.; Wang S.; Zhang M.; Dai J.; Xie J.; Zhu C. Deoxygenative Arylation of Carboxylic Acids by Aryl Migration. Chem.—Eur. J. 2019, 25, 12724–12729. 10.1002/chem.201903816. - DOI - PubMed
-
-
- Truce W. E.; Ray W. J. Jr; Norman O. L.; Eickemeyer D. B. Rearrangements of Aryl Sulfones. I. The metalation and rearrangement of mesityl phenyl sulfone. J. Am. Chem. Soc. 1958, 80, 3625–3629. 10.1021/ja01547a038. - DOI
-
See also:
- Naito T.; Dohmori R. Rearrangement of sulfonamide derivatives IV. Rearrangement reaction of the sulfonamide derivatives of pyridine and quinoline 1-oxides. Chem. Pharm. Bull. 1955, 3, 38–42. 10.1248/cpb1953.3.38. - DOI - PubMed
-
- Rabet P. T. G.; Boyd S.; Greaney M. F. Metal-Free Intermolecular Aminoarylation of Alkynes. Angew. Chem., Int. Ed. 2017, 56, 4183–4186. 10.1002/anie.201612445. - DOI - PubMed
- Barlow H. L.; Rabet P. T. G.; Durie A.; Evans T.; Greaney M. F. Arylation Using Sulfonamides: Phenylacetamide Synthesis through Tandem Acylation-Smiles Rearrangement. Org. Lett. 2019, 21, 9033–9035. 10.1021/acs.orglett.9b03429. - DOI - PubMed
- Johnson S.; Kovacs E.; Greaney M. F. Arylation and alkenylation of activated alkyl halides using sulfonamides. Chem. Commun. 2020, 56, 3222–3224. 10.1039/D0CC00220H. - DOI - PubMed
LinkOut - more resources
Full Text Sources

