The tRNA methyltransferase TrmB is critical for Acinetobacter baumannii stress responses and pulmonary infection
- PMID: 37589464
- PMCID: PMC10653896
- DOI: 10.1128/mbio.01416-23
The tRNA methyltransferase TrmB is critical for Acinetobacter baumannii stress responses and pulmonary infection
Abstract
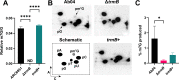
As deficiencies in tRNA modifications have been linked to human diseases such as cancer and diabetes, much research has focused on the modifications' impacts on translational regulation in eukaryotes. However, the significance of tRNA modifications in bacterial physiology remains largely unexplored. In this paper, we demonstrate that the m7G tRNA methyltransferase TrmB is crucial for a top-priority pathogen, Acinetobacter baumannii, to respond to stressors encountered during infection, including oxidative stress, low pH, and iron deprivation. We show that loss of TrmB dramatically attenuates a murine pulmonary infection. Given the current efforts to use another tRNA methyltransferase, TrmD, as an antimicrobial therapeutic target, we propose that TrmB, and other tRNA methyltransferases, may also be viable options for drug development to combat multidrug-resistant A. baumannii.
Keywords: Acinetobacter; iron acquisition; macrophages; oxidative stress; pneumonia; tRNA modification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

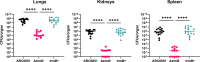
References
-
- Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. 2016. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2011-2014. Infect Control Hosp Epidemiol 37:1288–1301. doi: 10.1017/ice.2016.174 - DOI - PMC - PubMed
-
- Giammanco A, Calà C, Fasciana T, Dowzicky MJ, Bradford PA. 2017. Global assessment of the activity of tigecycline against multidrug-resistant gram-negative pathogens between 2004 and 2014 as part of the tigecycline evaluation and surveillance trial. mSphere 2:e00310-16. doi: 10.1128/mSphere.00310-16 - DOI - PMC - PubMed
-
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group . 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. doi: 10.1016/S1473-3099(17)30753-3 - DOI - PubMed
-
- CDC . 2022. COVID-19: U.S. Impact on Antimicrobial Resistance, SPECIAL REPORT 2022
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

