Enhancing autophagy in CD11c+ antigen-presenting cells as a therapeutic strategy for acute respiratory distress syndrome
- PMID: 37590140
- PMCID: PMC10510741
- DOI: 10.1016/j.celrep.2023.112990
Enhancing autophagy in CD11c+ antigen-presenting cells as a therapeutic strategy for acute respiratory distress syndrome
Abstract
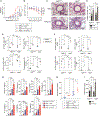
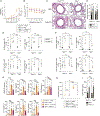
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are severe clinical disorders that mainly develop from viral respiratory infections, sepsis, and chest injury. Antigen-presenting cells play a pivotal role in propagating uncontrolled inflammation and injury through the excess secretion of pro-inflammatory cytokines and recruitment of immune cells. Autophagy, a homeostatic process that involves the degradation of cellular components, is involved in many processes including lung inflammation. Here, we use a polyinosinic-polycytidylic acid (poly(I:C))-induced lung injury mouse model to mimic viral-induced ALI/ARDS and show that disruption of autophagy in macrophages exacerbates lung inflammation and injury, whereas autophagy induction attenuates this process. Therefore, induction of autophagy in macrophages can be a promising therapeutic strategy in ALI/ARDS.
Keywords: CD11c; CP: Immunology; acute respiratory distress syndrome (ARDS); antigen-presenting cells; autophagy; lung inflammation; lung injury; poly(I:C).
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

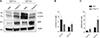


Similar articles
-
Progress in preclinical studies of macrophage autophagy in the regulation of ALI/ARDS.Front Immunol. 2022 Aug 18;13:922702. doi: 10.3389/fimmu.2022.922702. eCollection 2022. Front Immunol. 2022. PMID: 36059534 Free PMC article. Review.
-
LAIR-1 limits macrophage activation in acute inflammatory lung injury.Mucosal Immunol. 2023 Dec;16(6):788-800. doi: 10.1016/j.mucimm.2023.08.003. Epub 2023 Sep 10. Mucosal Immunol. 2023. PMID: 37634572 Free PMC article.
-
Role of cuproptosis in mediating the severity of experimental malaria-associated acute lung injury/acute respiratory distress syndrome.Parasit Vectors. 2024 Oct 19;17(1):433. doi: 10.1186/s13071-024-06520-1. Parasit Vectors. 2024. PMID: 39427197 Free PMC article.
-
Protective effect of β-glucan on Poly(I:C)-induced acute lung injury/inflammation: Therapeutic implications of viral infections in the respiratory system.Life Sci. 2023 Oct 1;330:122027. doi: 10.1016/j.lfs.2023.122027. Epub 2023 Aug 18. Life Sci. 2023. PMID: 37597767
-
MicroRNAs: Important Regulatory Molecules in Acute Lung Injury/Acute Respiratory Distress Syndrome.Int J Mol Sci. 2022 May 16;23(10):5545. doi: 10.3390/ijms23105545. Int J Mol Sci. 2022. PMID: 35628354 Free PMC article. Review.
Cited by
-
Pig Milk Exosome Packaging ssc-miR-22-3p Alleviates Pig Intestinal Epithelial Cell Injury and Inflammatory Response by Targeting MAPK14.Int J Mol Sci. 2024 Oct 5;25(19):10715. doi: 10.3390/ijms251910715. Int J Mol Sci. 2024. PMID: 39409044 Free PMC article.
-
Integrated bioinformatics and experimental analysis of mitochondrial-associated membrane function and mechanism in acute respiratory distress syndrome.Sci Rep. 2025 Jul 9;15(1):24602. doi: 10.1038/s41598-025-10405-3. Sci Rep. 2025. PMID: 40634559 Free PMC article.
-
Macrophage‑driven pathogenesis in acute lung injury/acute respiratory disease syndrome: Harnessing natural products for therapeutic interventions (Review).Mol Med Rep. 2025 Jan;31(1):16. doi: 10.3892/mmr.2024.13381. Epub 2024 Nov 8. Mol Med Rep. 2025. PMID: 39513609 Free PMC article. Review.
-
Hippo pathway and NLRP3-driven NETosis in macrophages: Mechanisms of viral pneumoniaaggravation.Cell Death Discov. 2025 Jul 14;11(1):323. doi: 10.1038/s41420-025-02556-z. Cell Death Discov. 2025. PMID: 40659620 Free PMC article.
-
CB2 stimulation of adipose resident ILC2s orchestrates immune balance and ameliorates type 2 diabetes mellitus.Cell Rep. 2024 Jul 23;43(7):114434. doi: 10.1016/j.celrep.2024.114434. Epub 2024 Jul 3. Cell Rep. 2024. PMID: 38963763 Free PMC article.
References
-
- Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, and Kuebler WM; Acute Lung Injury in Animals Study Group (2011). An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 44, 725–738. 10.1165/rcmb.2009-0210ST. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

