A green edge-hosted zinc single-site heterogeneous catalyst for superior Fenton-like activity
- PMID: 37590415
- PMCID: PMC10450848
- DOI: 10.1073/pnas.2221228120
A green edge-hosted zinc single-site heterogeneous catalyst for superior Fenton-like activity
Abstract
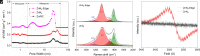
Developing green heterogeneous catalysts with excellent Fenton-like activity is critical for water remediation technologies. However, current catalysts often rely on toxic transitional metals, and their catalytic performance is far from satisfactory as alternatives of homogeneous Fenton-like catalysts. In this study, a green catalyst based on Zn single-atom was prepared in an ammonium atmosphere using ZIF-8 as a precursor. Multiple characterization analyses provided evidence that abundant intrinsic defects due to the edge sites were created, leading to the formation of a thermally stable edge-hosted Zn-N4 single-atom catalyst (ZnN4-Edge). Density functional theory calculations revealed that the edge sites equipped the single-atom Zn with a super catalytic performance, which not only promoted decomposition of peroxide molecule (HSO5-) but also greatly lowered the activation barrier for •OH generation. Consequently, the as-prepared ZnN4-Edge exhibited extremely high Fenton-like performance in oxidation and mineralization of phenol as a representative organic contaminant in a wide range of pH, realizing its quick detoxification. The atom-utilization efficiency of the ZnN4-Edge was ~104 higher than an equivalent amount of the control sample without edge sites (ZnN4), and the turnover frequency was ~103 times of the typical benchmark of homogeneous catalyst (Co2+). This study opens up a revolutionary way to rationally design and optimize heterogeneous catalysts to homogeneous catalytic performance for Fenton-like application.
Keywords: Fenton-like process; coordination environment; edge sites; peroxymonosulfate; single-atom catalysts.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Shannon M. A., et al. , Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008). - PubMed
-
- Gupta Sayam S., et al. , Rapid total destruction of chlorophenols by activated hydrogen peroxide. Science 296, 326–328 (2002). - PubMed
-
- Hodges B. C., Cates E. L., Kim J.-H., Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 13, 642–650 (2018). - PubMed
-
- Yang X.-J., Xu X.-M., Xu J., Han Y.-F., Iron oxychloride (FeOCl): An efficient Fenton-like catalyst for producing hydroxyl radicals in degradation of organic contaminants. J. Am. Chem. Soc. 135, 16058–16061 (2013). - PubMed
-
- Chen L., et al. , Strong enhancement on Fenton oxidation by addition of hydroxylamine to accelerate the ferric and ferrous iron cycles. Environ. Sci. Technol. 45, 3925–3930 (2011). - PubMed
Grants and funding
- 21JCZDJC00370/Natural Science Foundation of Tianjin City (Tianjin Natural Science Foundation)
- 20JCQNJC01840/Natural Science Foundation of Tianjin City (Tianjin Natural Science Foundation)
- 22106074/MOST | National Natural Science Foundation of China (NSFC)
- 41991313/MOST | National Natural Science Foundation of China (NSFC)
- 22111530176/MOST | National Natural Science Foundation of China (NSFC)
LinkOut - more resources
Full Text Sources

