Perioperative Chemoimmunotherapy With Durvalumab for Muscle-Invasive Urothelial Carcinoma: Primary Analysis of the Single-Arm Phase II Trial SAKK 06/17
- PMID: 37590894
- PMCID: PMC10666980
- DOI: 10.1200/JCO.23.00363
Perioperative Chemoimmunotherapy With Durvalumab for Muscle-Invasive Urothelial Carcinoma: Primary Analysis of the Single-Arm Phase II Trial SAKK 06/17
Abstract
Purpose: The integration of immunotherapy in the perioperative setting of muscle-invasive urothelial carcinoma (MIUC) appears promising. SAKK 06/17 investigated the addition of neoadjuvant durvalumab to gemcitabine/cisplatin (GC) chemotherapy followed by radical surgery and adjuvant checkpoint inhibition with durvalumab.
Patients and methods: SAKK 06/17 was an investigator-initiated, open-label, single-arm phase II study including cisplatin-fit patients with stage cT2-T4a cN0-1 operable MIUC. Four cycles of neoadjuvant GC in combination with four cycles of durvalumab (start with GC cycle 2) were administered, followed by radical surgery. Adjuvant durvalumab was given for 10 cycles. The primary end point was event-free survival (EFS) at 2 years.
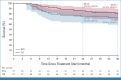
Results: Sixty one patients were accrued at 12 sites. The full analysis set consisted of 57 patients, 54 (95%) had bladder cancer. Median follow-up was 40 months. The primary end point was met, with EFS at 2 years of 76% (one-sided 90% CI [lower bound], 67%; two-sided 95% CI, 62 to 85). EFS at 3 years was 73% (95% CI, 59 to 83). Complete pathologic response in resected patients (N = 52) was achieved in 17 patients (33%), and 31 (60%) had pathologic response <ypT2 ypN0. Overall survival (OS) was 85% (95% CI, 72 to 92) at 2 years and 81% (95% CI, 67 to 89) at 3 years. Grade 3 and 4 treatment-related adverse events (TRAEs) during neoadjuvant treatment occurred in 42% and 25%, respectively. TRAEs related to adjuvant durvalumab were grade 3 in 5 (11%) and grade 4 in 2 (4%) patients.
Conclusion: The addition of perioperative durvalumab to the standard of care for patients with resectable MIUC results in a high EFS and OS at 2 years.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures



References
-
- International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group); European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer GroupAustralian Bladder Cancer Study Group, et al. : International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Long-term results of the BA06 30894 trial. J Clin Oncol 29:2171-2177, 2011 - PMC - PubMed
-
- Grossman HB, Natale RB, Tangen CM, et al. : Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349:859-866, 2003 - PubMed
-
- Vale CL: Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data: Advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 48:202-206, 2005 - PubMed
-
- Witjes JA, Bruins HM, Cathomas R, et al. : European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2020 guidelines. Eur Urol 79:82-104, 2021 - PubMed
-
- Pfister C, Gravis G, Fléchon A, et al. : Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin or gemcitabine and cisplatin as perioperative chemotherapy for patients with nonmetastatic muscle-invasive bladder cancer: Results of the GETUG-AFU V05 VESPER trial. J Clin Oncol 40:2013-2022, 2022 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

