APC and P53 mutations synergise to create a therapeutic vulnerability to NOTUM inhibition in advanced colorectal cancer
- PMID: 37591698
- PMCID: PMC10715527
- DOI: 10.1136/gutjnl-2022-329140
APC and P53 mutations synergise to create a therapeutic vulnerability to NOTUM inhibition in advanced colorectal cancer
Abstract
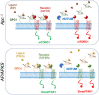
Objective: Colorectal cancer (CRC) is a leading cause of cancer-related deaths, with the majority of cases initiated by inactivation of the APC tumour suppressor. This results in the constitutive activation of canonical WNT pathway transcriptional effector ß-catenin, along with induction of WNT feedback inhibitors, including the extracellular palmitoleoyl-protein carboxylesterase NOTUM which antagonises WNT-FZD receptor-ligand interactions. Here, we sought to evaluate the effects of NOTUM activity on CRC as a function of driver mutation landscape.
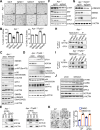
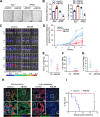
Design: Mouse and human colon organoids engineered with combinations of CRC driver mutations were used for Notum genetic gain-of-function and loss-of-function studies. In vitro assays, in vivo endoscope-guided orthotopic organoid implantation assays and transcriptomic profiling were employed to characterise the effects of Notum activity. Small molecule inhibitors of Notum activity were used in preclinical therapeutic proof-of-principle studies targeting oncogenic Notum activity.
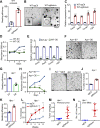
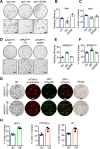
Results: NOTUM retains tumour suppressive activity in APC-null adenomas despite constitutive ß-catenin activity. Strikingly, on progression to adenocarcinoma with P53 loss, NOTUM becomes an obligate oncogene. These phenotypes are Wnt-independent, resulting from differential activity of NOTUM on glypican 1 and 4 in early-stage versus late-stage disease, respectively. Ultimately, preclinical mouse models and human organoid cultures demonstrate that pharmacological inhibition of NOTUM is highly effective in arresting primary adenocarcinoma growth and inhibiting metastatic colonisation of distal organs.
Conclusions: Our findings that a single agent targeting the extracellular enzyme NOTUM is effective in treating highly aggressive, metastatic adenocarcinomas in preclinical mouse models and human organoids make NOTUM and its glypican targets therapeutic vulnerabilities in advanced CRC.
Keywords: COLORECTAL CANCER; ONCOGENES.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Comment in
-
Pleiotropic role of NOTUM in colorectal cancer.Gut. 2023 Nov 24;72(12):2222-2223. doi: 10.1136/gutjnl-2023-330807. Gut. 2023. PMID: 37884353 Free PMC article. No abstract available.
References
-
- Miyaki M, Seki M, Okamoto M, et al. . Genetic changes and histopathological types in colorectal tumors from patients with familial adenomatous Polyposis. Cancer Res 1990;50:7166–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
