Tick extracellular vesicles in host skin immunity and pathogen transmission
- PMID: 37591719
- PMCID: PMC10528898
- DOI: 10.1016/j.pt.2023.07.009
Tick extracellular vesicles in host skin immunity and pathogen transmission
Abstract
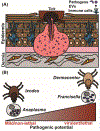
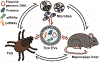
Ticks can transmit a variety of human pathogens, including intracellular and extracellular bacteria, viruses, and protozoan parasites. Historically, their saliva has been of immense interest due to its anticoagulant, anti-inflammatory, and anesthetic properties. Only recently, it was discovered that tick saliva contains extracellular vesicles (EVs). Briefly, it has been observed that proteins associated with EVs are important for multiple tick-borne intracellular microbial lifestyles. The impact of tick EVs on viral and intracellular bacterial pathogen transmission from the tick to the mammalian host has been shown experimentally. Additionally, tick EVs interact with the mammalian skin immune system at the bite site. The interplay between tick EVs, the transmission of pathogens, and the host skin immune system affords opportunities for future research.
Keywords: exosomes; microbial transmission; microvesicles; skin immunity; tick-borne disease.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests No interests are declared.
Figures

Similar articles
-
miRNA profile of extracellular vesicles isolated from saliva of Haemaphysalis longicornis tick.Acta Trop. 2020 Dec;212:105718. doi: 10.1016/j.actatropica.2020.105718. Epub 2020 Sep 21. Acta Trop. 2020. PMID: 32971070
-
Isolation of microRNAs from Tick Ex Vivo Salivary Gland Cultures and Extracellular Vesicles.J Vis Exp. 2022 Apr 6;(182). doi: 10.3791/63618. J Vis Exp. 2022. PMID: 35467650
-
Proteomic Analysis of Exosome-Like Vesicles Isolated From Saliva of the Tick Haemaphysalis longicornis.Front Cell Infect Microbiol. 2020 Oct 22;10:542319. doi: 10.3389/fcimb.2020.542319. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33194791 Free PMC article.
-
Changing the Recipe: Pathogen Directed Changes in Tick Saliva Components.Int J Environ Res Public Health. 2021 Feb 12;18(4):1806. doi: 10.3390/ijerph18041806. Int J Environ Res Public Health. 2021. PMID: 33673273 Free PMC article. Review.
-
Ticks' tricks: immunomodulatory effects of ixodid tick saliva at the cutaneous tick-host interface.Front Immunol. 2025 Mar 27;16:1520665. doi: 10.3389/fimmu.2025.1520665. eCollection 2025. Front Immunol. 2025. PMID: 40213541 Free PMC article. Review.
Cited by
-
A glimpse into the world of microRNAs and their putative roles in hard ticks.Front Cell Dev Biol. 2024 Sep 23;12:1460705. doi: 10.3389/fcell.2024.1460705. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39376631 Free PMC article. Review.
-
Overview of extracellular vesicles in pathogens with special focus on human extracellular protozoan parasites.Mem Inst Oswaldo Cruz. 2024 Sep 23;119:e240073. doi: 10.1590/0074-02760240073. eCollection 2024. Mem Inst Oswaldo Cruz. 2024. PMID: 39319874 Free PMC article. Review.
-
Tick exposure biomarkers: A One Health approach to new tick surveillance tools.Curr Res Parasitol Vector Borne Dis. 2024 Aug 24;6:100212. doi: 10.1016/j.crpvbd.2024.100212. eCollection 2024. Curr Res Parasitol Vector Borne Dis. 2024. PMID: 39286798 Free PMC article.
-
Extracellular vesicles in arbovirus infections: from basic biology to potential clinical applications.Front Cell Infect Microbiol. 2025 Apr 28;15:1558520. doi: 10.3389/fcimb.2025.1558520. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40357393 Free PMC article. Review.
-
mGem: Decoding transmicrobe messaging-the growing impact of extracellular vesicles.mBio. 2025 Jun 11;16(6):e0313024. doi: 10.1128/mbio.03130-24. Epub 2025 Apr 29. mBio. 2025. PMID: 40298402 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

