A novel antifolate suppresses growth of FPGS-deficient cells and overcomes methotrexate resistance
- PMID: 37591722
- PMCID: PMC10435995
- DOI: 10.26508/lsa.202302058
A novel antifolate suppresses growth of FPGS-deficient cells and overcomes methotrexate resistance
Abstract
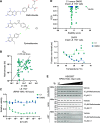
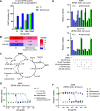
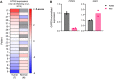
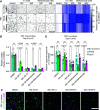
Cancer cells make extensive use of the folate cycle to sustain increased anabolic metabolism. Multiple chemotherapeutic drugs interfere with the folate cycle, including methotrexate and 5-fluorouracil that are commonly applied for the treatment of leukemia and colorectal cancer (CRC), respectively. Despite high success rates, therapy-induced resistance causes relapse at later disease stages. Depletion of folylpolyglutamate synthetase (FPGS), which normally promotes intracellular accumulation and activity of natural folates and methotrexate, is linked to methotrexate and 5-fluorouracil resistance and its association with relapse illustrates the need for improved intervention strategies. Here, we describe a novel antifolate (C1) that, like methotrexate, potently inhibits dihydrofolate reductase and downstream one-carbon metabolism. Contrary to methotrexate, C1 displays optimal efficacy in FPGS-deficient contexts, due to decreased competition with intracellular folates for interaction with dihydrofolate reductase. We show that FPGS-deficient patient-derived CRC organoids display enhanced sensitivity to C1, whereas FPGS-high CRC organoids are more sensitive to methotrexate. Our results argue that polyglutamylation-independent antifolates can be applied to exert selective pressure on FPGS-deficient cells during chemotherapy, using a vulnerability created by polyglutamylation deficiency.
© 2023 van der Krift et al.
Conflict of interest statement
MM Maurice is an inventor on patents related to membrane protein degradation; she is co-founder and shareholder of Laigo Bio.
Figures








References
-
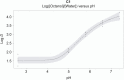
- Andrés A, Rosés M, Ràfols C, Bosch E, Espinosa S, Segarra V, Huerta JM (2015) Setup and validation of shake-flask procedures for the determination of partition coefficients (log D) from low drug amounts. Eur J Pharm Sci official J Eur Fed Pharm Sci 76: 181–191. 10.1016/j.ejps.2015.05.008 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
