Monoclonal Antibodies: What the Diagnostic Neuroradiologist Needs to Know
- PMID: 37591772
- PMCID: PMC10714862
- DOI: 10.3174/ajnr.A7974
Monoclonal Antibodies: What the Diagnostic Neuroradiologist Needs to Know
Abstract
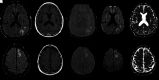
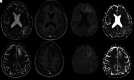
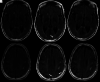
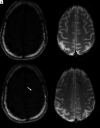
Monoclonal antibodies have become increasingly popular as novel therapeutics against a variety of diseases due to their specificity, affinity, and serum stability. Due to the nearly infinite repertoire of monoclonal antibodies, their therapeutic use is rapidly expanding, revolutionizing disease course and management, and what is now considered experimental therapy may soon become approved practice. Therefore, it is important for radiologists, neuroradiologists, and neurologists to be aware of these drugs and their possible different imaging-related manifestations, including expected and adverse effects of these novel drugs. Herein, we review the most commonly used monoclonal antibody-targeted therapeutic agents, their mechanism of action, clinical applications, and major adverse events with a focus on neurologic and neurographic effects and discuss differential considerations, to assist in the diagnosis of these conditions.
© 2023 by American Journal of Neuroradiology.
Figures

Similar articles
-
Monoclonal antibody-based molecular imaging strategies and theranostic opportunities.Theranostics. 2020 Jan 1;10(2):938-955. doi: 10.7150/thno.37443. eCollection 2020. Theranostics. 2020. PMID: 31903161 Free PMC article. Review.
-
Approved monoclonal antibodies for cancer therapy.Expert Opin Biol Ther. 2008 Aug;8(8):1151-8. doi: 10.1517/14712598.8.8.1151. Expert Opin Biol Ther. 2008. PMID: 18613766 Review.
-
Production, novel assay development and clinical applications of monoclonal antibodies.Recent Pat Anticancer Drug Discov. 2011 May;6(2):258-67. doi: 10.2174/157489211795328549. Recent Pat Anticancer Drug Discov. 2011. PMID: 21247405 Review.
-
Monoclonal antibodies and side-effect management.Oncology (Williston Park). 2006 Sep;20(10 Suppl Nurse Ed):11-21; discussion 22-3. Oncology (Williston Park). 2006. PMID: 18153975 Review.
-
Biopharmaceuticals and monoclonal antibodies in oncology trials--a cross-sectional analysis.Protein Eng Des Sel. 2011 Jan;24(1-2):105-11. doi: 10.1093/protein/gzq090. Epub 2010 Oct 30. Protein Eng Des Sel. 2011. PMID: 21037277 Free PMC article.
References
-
- Sanderson K, Scotland R, Lee P, et al. . Autoimmunity in a Phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol 2005;23:741–50 10.1200/JCO.2005.01.128 - DOI - PubMed
