Gabapentinoid consumption in 65 countries and regions from 2008 to 2018: a longitudinal trend study
- PMID: 37591833
- PMCID: PMC10435503
- DOI: 10.1038/s41467-023-40637-8
Gabapentinoid consumption in 65 countries and regions from 2008 to 2018: a longitudinal trend study
Abstract
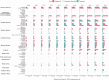
Recent studies raised concerns about the increasing use of gabapentinoids in different countries. With their potential for misuse and addiction, understanding the global consumption of gabapentinoids will offer us a platform to examine the need for any interventional policies. This longitudinal trend study utilised pharmaceutical sales data from 65 countries and regions across the world to evaluate the global trends in gabapentinoid consumption between 2008-2018. The multinational average annual percentage change of gabapentinoid consumption was +17.20%, increased from 4.17 defined daily dose per ten thousand inhabitants per day (DDD/TID) in 2008 to 18.26 DDD/TID in 2018. High-income countries had the highest pooled gabapentinoid consumption rate (39.92 DDD/TID) in 2018, which was more than six times higher than the lower-middle income countries (6.11 DDD/TID). The study shows that despite differences in healthcare system and culture, a consistent increase in gabapentinoid consumption is observed worldwide, with high-income countries remaining the largest consumers.
© 2023. Springer Nature Limited.
Conflict of interest statement
A.Y.L.C. is supported by the AIR@innoHK programme of the Hong Kong Innovation and Technology Commission. D.P.J.O and J.F.H are supported by the University College London Hospitals NIHR Biomedical Research Centre and the NIHR North Thames Applied Research Collaboration. I.C.K.W. received research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, Takeda, the Hong Kong Research Grants Council, and the Hong Kong Health and Medical Research Fund, National Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia. He is also a non-executive director of Jacobson Pharma Corporation Limited in Hong Kong and a consultant to the World Health Organization. K.K.C.M. reports grants from the CW Maplethorpe Fellowship, the European Union Horizon 2020, the UK National Institute of Health Research and the Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, and reports personal fees from IQVIA, unrelated to the submitted work. The remaining authors declare no competing interests. All funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The views expressed in this article are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Figures



References
-
- Goodman CW, Brett AS. Gabapentin and pregabalin for pain—Is increased prescribing a cause for concern? N. Engl. J. Med. 2017;377:411–414. - PubMed
-
- Goodman CW, Brett AS. A clinical overview of off-label use of gabapentinoid drugs. JAMA Intern. Med. 2019;179:695–701. - PubMed
-
- Joint Formulary Committee. British National Formulary: Gabapentin (BMJ Group and Pharmaceutical Press, 2022).
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

