VEGF-B prevents excessive angiogenesis by inhibiting FGF2/FGFR1 pathway
- PMID: 37591843
- PMCID: PMC10435562
- DOI: 10.1038/s41392-023-01539-9
VEGF-B prevents excessive angiogenesis by inhibiting FGF2/FGFR1 pathway
Abstract
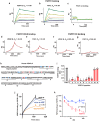
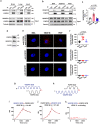
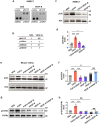
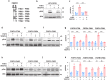
Although VEGF-B was discovered as a VEGF-A homolog a long time ago, the angiogenic effect of VEGF-B remains poorly understood with limited and diverse findings from different groups. Notwithstanding, drugs that inhibit VEGF-B together with other VEGF family members are being used to treat patients with various neovascular diseases. It is therefore critical to have a better understanding of the angiogenic effect of VEGF-B and the underlying mechanisms. Using comprehensive in vitro and in vivo methods and models, we reveal here for the first time an unexpected and surprising function of VEGF-B as an endogenous inhibitor of angiogenesis by inhibiting the FGF2/FGFR1 pathway when the latter is abundantly expressed. Mechanistically, we unveil that VEGF-B binds to FGFR1, induces FGFR1/VEGFR1 complex formation, and suppresses FGF2-induced Erk activation, and inhibits FGF2-driven angiogenesis and tumor growth. Our work uncovers a previously unrecognized novel function of VEGF-B in tethering the FGF2/FGFR1 pathway. Given the anti-angiogenic nature of VEGF-B under conditions of high FGF2/FGFR1 levels, caution is warranted when modulating VEGF-B activity to treat neovascular diseases.
© 2023. West China Hospital, Sichuan University.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Nag S, Eskandarian MR, Davis J, Eubanks JH. Differential expression of vascular endothelial growth factor-A (VEGF-A) and VEGF-B after brain injury. J. Neuropathol. Exp. Neurol. 2002;61:778–788. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

