Long-term outcomes of allogeneic stem cell transplant in multiple myeloma
- PMID: 37591876
- PMCID: PMC10435482
- DOI: 10.1038/s41408-023-00900-z
Long-term outcomes of allogeneic stem cell transplant in multiple myeloma
Abstract
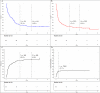
Allogeneic stem cell transplant (allo SCT) for multiple myeloma (MM) is potentially curative in some, while toxic in many others. We retrospectively analyzed 85 patients diagnosed with MM who underwent allo SCT as frontline or salvage therapy between 2000 and 2022 at Mayo Clinic Rochester and examined patient outcomes and prognostic markers. Overall survival (OS), progression free survival (PFS), treatment related mortality (TRM), and relapse rates (RR) were estimated using the Kaplan Meier method and competing risk models. Median follow-up was 11.5 years. Median OS and PFS were 1.7 and 0.71 years, respectively. Five-year OS and PFS were 22.2% and 15.1%, respectively. One-year TRM was 23.5%. Twelve patients demonstrated durable overall survival, living 10+ years beyond their allo SCT. This subgroup was more likely to have no or one prior auto SCT (p = 0.03) and to have been transplanted between 2000 and 2010 (p = 0.03). Outcomes were poor in this cohort with long follow-up, with few patients surviving 5 years or more, and most relapsing or dying within 2 years. We would expect better outcomes and tolerability with an expanded array of novel therapeutics and would prefer them to allo SCT.
© 2023. Springer Nature Limited.
Conflict of interest statement
SKK has received research funding from and holds membership on advisory boards for AbbVie, Celgene, Janssen, Takeda, Adaptive, MedImmune, and has received research funding from KITE, Merck, Novartis, Roche, and Sanofi. AD holds membership on advisory boards from Janssen and research funding from Alynlam, Pfizer, Takeda, and BMS. DD has performed consultancy for Alexion, Apellis, Bristol Meyers Squibb, Novartis, Roche, and Sanofi, and research funding from Regeneron. MQL has received research funding from Celgene. PK has received honoraria and research funding from Sanofi, BMS, and AbbVie, research funding from Regeneron, Amgen, Ichnos, Loxo, Karyopharma, and Takeda, and honoraria from Casma, Pharmacyclics, Imedex, GSK, Cellectar, Oncopeptides, and X4. NL Holds a membership on advisory boards for Takeda. EM has received honoraria from Janssen and performs consultancy for Protego. TK has received research funding from Novartis. MAG has received fees from Ionis/Akcea, Prothena, Sanofi, Janssen, Aptitude, Ashfield, Juno, Physician’s Education Resource, Abbvie, J&J, Research to Practice, Celgene, and Sorrento, and has developed education materials for i3Health.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

