Inactivation kinetics of horseradish peroxidase (HRP) by hydrogen peroxide
- PMID: 37591893
- PMCID: PMC10435507
- DOI: 10.1038/s41598-023-39687-1
Inactivation kinetics of horseradish peroxidase (HRP) by hydrogen peroxide
Abstract
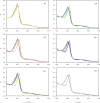
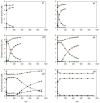
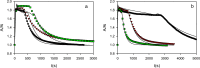
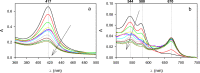
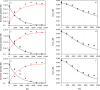
In recent years, the peroxidase enzymes have generated wide interest in several industrial processes, such as wastewater treatments, food processing, pharmaceuticals, and the production of fine chemicals. However, the low stability of the peroxidases in the presence of hydrogen peroxide (H2O2) has limited its commercial use. In the present work, the effect of H2O2 on the inactivation of horseradish peroxidase (HRP) was evaluated. Three states of HRP (E0, E2, and E3) were identified. While in the absence of H2O2, the resting state E0 was observed, in the presence of low and high concentrations of H2O2, E2, and E3 were found, respectively. The results showed that HRP catalyzed the H2O2 decomposition, forming the species Ex, which was catalytically inactive. Results suggest that this loss of enzymatic activity is an intrinsic characteristic of the studied HRP. A model from a modified version of the Dunford mechanism of peroxidases was developed, which was validated against experimental data and findings reported by the literature.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Chiong T, Lau SY, Lek ZH, Koh BY, Danquah MK. Enzymatic treatment of methyl orange dye in synthetic wastewater by plant-based peroxidase enzymes. J. Environ. Chem. Eng. 2016;4:2500–2509. doi: 10.1016/j.jece.2016.04.030. - DOI
-
- Darwesh, O.M.; Matter, I.A.; EidaM, .F Application of Nanotechnology to Stabilize Peroxidase Enzyme and Improve It in Textile Dyes Removal from Wastewater. In Proceedings of the Conference on Water Resources and Arid Environments ; 2016; pp. 465–473.
-
- Ayyagari M, Akkara JA, Kaplan DL. Enzyme-mediated polymerization reactions: Peroxidase-catalyzed polyphenol synthesis. Acta Polym. 1996;47:193–203. doi: 10.1002/actp.1996.010470501. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

