Clinical impact of the genomic landscape and leukemogenic trajectories in non-intensively treated elderly acute myeloid leukemia patients
- PMID: 37591941
- PMCID: PMC10624608
- DOI: 10.1038/s41375-023-01999-6
Clinical impact of the genomic landscape and leukemogenic trajectories in non-intensively treated elderly acute myeloid leukemia patients
Erratum in
-
Correction: Clinical impact of the genomic landscape and leukemogenic trajectories in non-intensively treated elderly acute myeloid leukemia patients.Leukemia. 2023 Nov;37(11):2336-2337. doi: 10.1038/s41375-023-02017-5. Leukemia. 2023. PMID: 37789148 Free PMC article. No abstract available.
Abstract
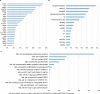
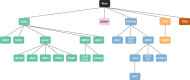
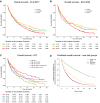
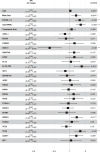
To characterize the genomic landscape and leukemogenic pathways of older, newly diagnosed, non-intensively treated patients with AML and to study the clinical implications, comprehensive genetics analyses were performed including targeted DNA sequencing of 263 genes in 604 patients treated in a prospective Phase III clinical trial. Leukemic trajectories were delineated using oncogenetic tree modeling and hierarchical clustering, and prognostic groups were derived from multivariable Cox regression models. Clonal hematopoiesis-related genes (ASXL1, TET2, SRSF2, DNMT3A) were most frequently mutated. The oncogenetic modeling algorithm produced a tree with five branches with ASXL1, DDX41, DNMT3A, TET2, and TP53 emanating from the root suggesting leukemia-initiating events which gave rise to further subbranches with distinct subclones. Unsupervised clustering mirrored the genetic groups identified by the tree model. Multivariable analysis identified FLT3 internal tandem duplications (ITD), SRSF2, and TP53 mutations as poor prognostic factors, while DDX41 mutations exerted an exceptionally favorable effect. Subsequent backwards elimination based on the Akaike information criterion delineated three genetic risk groups: DDX41 mutations (favorable-risk), DDX41wildtype/FLT3-ITDneg/TP53wildtype (intermediate-risk), and FLT3-ITD or TP53 mutations (high-risk). Our data identified distinct trajectories of leukemia development in older AML patients and provide a basis for a clinically meaningful genetic outcome stratification for patients receiving less intensive therapies.
© 2023. The Author(s).
Conflict of interest statement
EJ: Employment with AstraZeneca; MS, MG, PL, AR, CP, NJ, CW, KH, NS, JK, AB, SN have nothing to disclose; PF: Honoraria from BMS, Novartis, Jazz, Abbvie, Agios, Servier, Research support (as Groupe Francophone des Myélodysplasies chairperson) : BMS, Novartis, Jazz, Abbvie, Agios, Servier, GJR: Consultancy: Abbvie, Amgen, Argenx, AstraZeneca, Bluebird Bio, Blueprint Medicines, Bristol-Myers Squibb, Caribou Biosciences, Celgene, Daiichi Sankyo, Ellipses Pharma, GlaxoSmithKline, Janssen, Jasper Pharmaceuticals, Jazz Pharmaceuticals, Molecular Partners, Novartis, Pfizer, Roche, Syndax, Takeda (IRC Chair); LB advisory role or expert testimony – Abbvie, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Gilead, Hexal, Janssen, Jazz Pharmaceuticals, Menarini, Novartis, Pfizer; Honoraria – Abbvie, Amgen, Astellas, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Janssen, Jazz Pharmaceuticals, Novartis, Pfizer, Sanofi; Financing of scientific research – Bayer, Jazz Pharmaceuticals. HNK: Employment with Astex Pharmaceuticals, Inc.; YH, MA: Consultancy for Astex Pharmaceuticals, Inc.; KD: Advisory role for Amgen, Bristol-Myers Squibb, Celgene, Janssen, Jazz Pharmaceuticals, Novartis, Roche, Daiichi Sankyo; research funding from: Agios, Astex, Astellas, Bristol-Myers Squibb, Celgene, Kronos-Bio, Novartis; HD: Advisory role for Abbvie, Agios, Amgen, Astellas, AstraZeneca, Berlin-Chemie, Bristol-Myers Squibb, Celgene, GEMoaB, Gilead, Janssen, Jazz Pharmaceuticals, Novartis, Syndax; research funding from Abbvie, Agios, Amgen, Astellas, Bristol-Myers Squibb, Jazz Pharmaceuticals, Kronos-Bio, Novartis.
Figures

References
-
- SEER, Cancer Stat Facts: acute myeloid leukemia. Bethesda, MD: National Cancer Institute. https://seer.cancer.gov/statfacts/html/amyl.html.
-
- Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–7. doi: 10.1200/JCO.2011.38.9429. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

