Core protocol development for phase 2/3 clinical trials in the leukodystrophy vanishing white matter: a consensus statement by the VWM consortium and patient advocates
- PMID: 37592248
- PMCID: PMC10433679
- DOI: 10.1186/s12883-023-03354-9
Core protocol development for phase 2/3 clinical trials in the leukodystrophy vanishing white matter: a consensus statement by the VWM consortium and patient advocates
Abstract
Background: The leukodystrophy "Vanishing White Matter" (VWM) is an orphan disease with neurological decline and high mortality. Currently, VWM has no approved treatments, but advances in understanding pathophysiology have led to identification of promising therapies. Several investigational medicinal products are either in or about to enter clinical trial phase. Clinical trials in VWM pose serious challenges, as VWM has an episodic disease course; disease phenotype is highly heterogeneous and predictable only for early onset; and study power is limited by the small patient numbers. To address these challenges and accelerate therapy delivery, the VWM Consortium, a group of academic clinicians with expertise in VWM, decided to develop a core protocol to function as a template for trials, to improve trial design and facilitate sharing of control data, while permitting flexibility regarding other trial details. Overall aims of the core protocol are to collect safety, tolerability, and efficacy data for treatment assessment and marketing authorization.
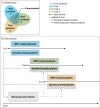
Methods: To develop the core protocol, the VWM Consortium designated a committee, including clinician members of the VWM Consortium, family and patient group advocates, and experts in statistics, clinical trial design and alliancing with industries. We drafted three age-specific protocols, to stratify into more homogeneous patient groups, of ages ≥ 18 years, ≥ 6 to < 18 years and < 6 years. We chose double-blind, randomized, placebo-controlled design for patients aged ≥ 6 years; and open-label non-randomized natural-history-controlled design for patients < 6 years. The protocol describes study populations, age-specific endpoints, inclusion and exclusion criteria, study schedules, sample size determinations, and statistical considerations.
Discussion: The core protocol provides a shared uniformity across trials, enables a pool of shared controls, and reduces the total number of patients necessary per trial, limiting the number of patients on placebo. All VWM clinical trials are suggested to adhere to the core protocol. Other trial components such as choice of primary outcome, pharmacokinetics, pharmacodynamics, and biomarkers are flexible and unconstrained by the core protocol. Each sponsor is responsible for their trial execution, while the control data are handled by a shared research organization. This core protocol benefits the efficiency of parallel and consecutive trials in VWM, and we hope accelerates time to availability of treatments for VWM.
Trial registration: NA. From a scientific and ethical perspective, it is strongly recommended that all interventional trials using this core protocol are registered in a clinical trial register.
Keywords: Core protocol; Innovative trial design; Leukodystrophy; Orphan disease; Trial protocol; Vanishing white matter.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
AV: receives funding or in kind support for research from Illumina, Eli Lilly, Takeda, Sana, Affinyia, Sanofi, Passage Bio, Ionis, Homology, Myrtelle, Biogen, Boehringer Ingelheim, Synaptix Bio, without personal compensation.
AF: Institutional consultant to Poxel Therapeutics, SwanBio Therapeutics, Adonis Therapeutics. Clinical Trial Site Principal Investigator for Minoryx, and Viking Therapeutics. Coinventor on patent licensed to Ashvattha Therapeutics.
BF: The institution of BF has received research support from the National Institutes of Health and the National Ataxia Foundation.
EB: co-investigator for trial in Alexander Leukodystrophy (IONIS) with no personal payment.
GB: consultant for Passage Bio Inc (2020–2022) and Ionis (2019); site investigator for the Alexander’s disease trial of Ionis (2021-present), Metachromatic leukodystrophy of Shire/Takeda (2020–2021), Krabbe and GM1 gene therapy trials of Passage Bio (2021-present), GM1 natural history study from the University of Pennsylvania sponsored by Passage Bio (2021-present) and Adrenoleukodystrophy/Hematopoietic stem cell transplantation natural history study of Bluebird Bio (2019); site sub-investigator for the MPS II gene therapy trial of Regenxbio (2021-present) and the MPS II clinical trial of Denali (2022-present); received an unrestricted educational grant from Takeda (2021–2022).
IKM: advisor for trials in Metachromatic Leukodystrophy and other leukodystrophies (Shire/Takeda, Orchard, PassageBio), without personal payment.
JLB: co-investigator for trials in MLD (Shire/Takeda); Krabbe disease (PassageBio); and VWM (Calico), without personal payment. Writer of content for UpToDate on MLD and VWM.
MSvdK: consultant for Calico (VWM) and co-investigator for Ionis (Alexander disease trial), without personal payment. She is on patent P112686US00 “therapeutic effects of Guanabenz treatment in vanishing white matter” and on patent P112686CA00 “the use of Guanabenz in the treatment of VWM”, both for the VU University Medical Center, Amsterdam, The Netherlands. She is the initiator and principal investigator of the Guanabenz trial (
NIW: advisor and/or co-investigator for trials in Metachromatic Leukodystrophy, Pelizaeus-Merzbacher disease and other leukodystrophies (Shire/Takeda, Orchard, Ionis, PassageBio, VigilNeuro, Sana Biotechnology, Lilly), without personal payment.
All other authors (AB, BH, DHS, DS, JB, PSL, EFS-V, RR, HD, PvB, MDS) have nothing to declare.
Figures

References
-
- Stellingwerff MD, Al-Saady ML, van de Brug T, Barkhof F, Pouwels PJW, van der Knaap MS. MRI natural history of the leukodystrophy vanishing white matter. Radiology. 2021;300(3):671–680. - PubMed
-
- Fogli A, Schiffmann R, Bertini E, Ughetto S, Combes P, Eymard-Pierre E, et al. The effect of genotype on the natural history of eIF2B-related leukodystrophies. Neurology. 2004;62(9):1509–1517. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

