Membranes for Oil/Water Separation: A Review
- PMID: 37593153
- PMCID: PMC10428143
- DOI: 10.1002/admi.202200557
Membranes for Oil/Water Separation: A Review
Abstract
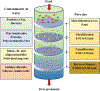
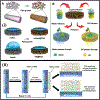
Recent advancements in separation and membrane technologies have shown a great potential in removing oil from wastewaters effectively. In addition, the capabilities have improved to fabricate membranes with tunable properties in terms of their wettability, permeability, antifouling, and mechanical properties that govern the treatment of oily wastewaters. Herein, authors have critically reviewed the literature on membrane technology for oil/water separation with a specific focus on: 1) membrane properties and characterization, 2) development of various materials (e.g., organic, inorganic, and hybrid membranes, and innovative materials), 3) membranes design (e.g., mixed matrix nanocomposite and multilayers), and 4) membrane fabrication techniques and surface modification techniques. The current challenges and future research directions in materials and fabrication techniques for membrane technology applications in oil/water separation are also highlighted. Thus, this review provides helpful guidance toward finding more effective, practical, and scalable solutions to tackle environmental pollution by oils.
Keywords: antifouling; membrane design; membrane materials; membrane technologies; oil/water separation.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest.
Figures
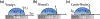















References
-
- Dave S, Sharma R, Int. J. Environ. Sci. Technol 2015, 4, 103.
-
- Islam MS, McCutcheon JR, Rahaman MS, J. Mater. Sci 2017, 537, 297.
-
- Lin X, Hong J, Adv. Mater. Interfaces 2019, 6, 1900126.
-
- Li X, Zhang C, Liu J, Min. Sci. Technol 2010, 20, 778.
-
- Al-Shamrani AA, James A, Xiao H, Water Res. 2002, 36, 1503. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
