Ketamine for the treatment of major depression: a systematic review and meta-analysis
- PMID: 37593223
- PMCID: PMC10430179
- DOI: 10.1016/j.eclinm.2023.102127
Ketamine for the treatment of major depression: a systematic review and meta-analysis
Abstract
Background: Intranasal esketamine has received regulatory approvals for the treatment of depression. Recently a large trial of repeated dose racemic ketamine also demonstrated efficacy in severe depression. However, uncertainties remain regarding comparative efficacy, dosage, and the time course of response.
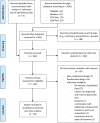
Methods: In this systematic review and meta-analysis, we searched Embase, Medline, Pubmed, PsycINFO, and CENTRAL up to April 13, 2023, for randomised controlled trials (RCTs) investigating ketamine for depression. Two investigators independently assessed study eligibility and risk of bias and extracted the data on depression severity scores, response and remission rates, and all-cause dropouts. Multivariable mixed-effects meta-regressions incorporated drug formulation (racemic (Rac) or esketamine (Esket)) and dose (Low or High) as covariates. Treatment effects were assessed: immediately following the first dose, during further repeated dosing, and follow-up after the final dose of a treatment course. This study is registered with PROSPERO (CRD42021221157).
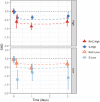
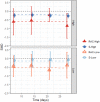
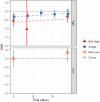
Findings: The systematic review identified 687 articles, of which 49 RCTs were eligible for analysis, comprising 3299 participants. Standardised mean differences (95% confidence intervals) immediately following the first/single treatment were moderate-high for all conditions (Rac-High: -0.73, -0.91 to -0.56; Esket-High: -0.48, -0.75 to -0.20; Rac-Low: -0.33, -0.54 to -0.12; Esket-Low: -0.55, -0.87 to -0.24). Ongoing effects during repeated dosing were significantly greater than the control for Rac-High (-0.61; -1.02 to -0.20) and Rac-Low (-0.55, -1.09 to -0.00), but not Esket-Low (-0.15, -0.49 to 0.19) or Esket-High (-0.22, -0.54 to 0.10). At follow-up effects remained significant for racemic ketamine (-0.65; -1.23 to -0.07) but not esketamine (-0.33; -0.96 to 0.31). All-cause dropout was similar between experiment and control conditions for both formulations combined (Odds Ratio = 1.18, 0.85-1.64). Overall heterogeneity varied from 5.7% to 87.6.
Interpretation: Our findings suggested that effect sizes for depression severity, as well as response and remission rates, were numerically greater for racemic ketamine than esketamine. Higher doses were more effective than low doses. Differences were evident in initial effects, ongoing treatment, and lasting effects after the final dose.
Funding: None.
Keywords: Depression; Ketamine; Meta-analysis; Systematic review.
© 2023 The Authors.
Conflict of interest statement
AB has been awarded doctoral studies research funding from the Canadian Institutes of Health Research Fellowship and research funding through the Calgary Health Trust. AB receives a small honorarium for teaching undergraduate and postgraduate medical trainees in the Cumming School of Medicine at the University of Calgary. AB is an unpaid member of the Canadian Network for Mood and Anxiety Treatments editorial committee, the International Society of Addiction Journal Editors, the Canadian Society of Addiction Medicine policy committee, and the Addiction Psychiatry section of the Canadian Psychiatric Association. AB is also an unpaid associate editor of the Canadian Journal of Addiction and a mental health educator for TED-Ed, where he receives a small honorarium for supporting online educational content. CZ is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R, 6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydroxylated and hydroxylated metabolites of (R, S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R, 6R)-hydroxynorketamine and (2S, 6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties the government may receive. GV has received grants from the Canadian Institute of Health Research; Canadian Biomarker Integration Network in Depression; Canadian Ontario Ministry of Health and Long-Term Care; Queen's University Medical School (RIG and Dean's Doctoral Award); Research Innovation Fund (Providence Care Hospital); Women's Giving Circle UHKK. GV is a speaker or member of the advisory board for: Abbvie, Allergan, Janssen, Lundbeck/Otsuka, NeonMind Biosciences, Asofarma, Raffo, Gador, Eurofarma, Elea/Phoenix, Psicofarma, Tecnofarma, Sunovion, Janssen. CL is supported by an NHMRC (Australian National Health and Medical Council) investigator Grant (1195651). CL has served on an advisory board for Janssen Cilag and a scientific advisory committee for Douglas Pharmaceuticals. She is the Medical Director of Neurostimulation/Interventional Psychiatry at the Ramsay Northside Clinic. The remaining authors have no conflicts of interest to declare.
Figures
References
-
- Lee E.E., Della Selva M.P., Liu A., Himelhoch S. Ketamine as a novel treatment for major depressive disorder and bipolar depression: a systematic review and quantitative meta-analysis. Gen Hosp Psychiatry. 2015;37(2):178–184. - PubMed
-
- Conley A.A., Norwood A.E., Hatvany T.C., Griffith J.D., Barber K.E. Efficacy of ketamine for major depressive episodes at 2, 4, and 6-weeks post-treatment: a meta-analysis. Psychopharmacology. 2021;238(7):1737–1752. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

