Homologous versus Heterologous prime-boost COVID-19 Vaccination in autologous hematopoietic stem cell transplantation recipients: a blinded randomized controlled trial
- PMID: 37593732
- PMCID: PMC10427916
- DOI: 10.3389/fimmu.2023.1237916
Homologous versus Heterologous prime-boost COVID-19 Vaccination in autologous hematopoietic stem cell transplantation recipients: a blinded randomized controlled trial
Abstract
Background/purpose: Optimizing vaccine efficacy is of particular concern in patients undergoing hematopoietic stem cell transplantation (HSCT), which mainly have an inadequate immune response to primary SARS-CoV-2 vaccination. This investigation aimed to explore the potential prime-boost COVID-19 vaccination strategies following autologous (auto-) HSCT.
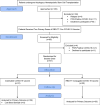
Methods: In a randomized clinical trial, patients who had already received two primary doses of receptor-binding domain (RBD) tetanus toxoid (TT) conjugated SARS-CoV-2 vaccine during three to nine months after auto-HSCT were randomized to receive either a homologous RBD-TT conjugated or heterologous inactivated booster dose four weeks after the primary vaccination course. The primary outcome was comparing the anti-S IgG Immune status ratio (ISR) four weeks after the heterologous versus homologous booster dose. The assessment of safety and reactogenicity adverse events was considered as the secondary outcome.
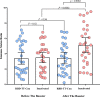
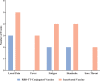
Results: Sixty-one auto-HSCT recipients were recruited and randomly assigned to receive either homologous or heterologous booster doses four weeks after the primary vaccination course. The mean ISR was 3.40 (95% CI: 2.63- 4.16) before the booster dose with a 90.0% seropositive rate. The ISR raised to 5.12 (95% CI: 4.15- 6.08) with a 100% seropositive rate after heterologous (P= 0.0064) and to 3.42 (95% CI: 2.67- 4.17) with a 93.0% seropositivity after the homologous booster doses (P= 0.96). In addition, the heterologous group suffered more AEs following the booster dosage than the homologous group, but this difference was not statistically significant (p = 0.955). In multivariable analysis, the prime-boost vaccination strategy (heterologous versus homologous), the level of ISR before the booster dose, and the length of time between auto-HSCT and booster dose were the positive predictors of serologic response to a booster dose. No serious adverse event is attributed to booster vaccination.
Conclusion: In patients who were primed with two SARS-CoV-2 vaccine doses during the first year after auto-HSCT, heterologous prime-boost COVID-19 vaccination with inactivated platform resulted in considerably enhanced serologic response and non-significantly higher reactogenicity adverse events than homologous RBD-TT conjugated prime-boost COVID-19 vaccination strategy.
Keywords: RBD subunit vaccine; SARS-CoV-2; hematopoietic stem cell transplantation; heterologous prime boost COVID-19 vaccination; immunogenicity; inactivated vaccines.
Copyright © 2023 Sharifi Aliabadi, Karami, Barkhordar, Hashemi Nazari, Kavousi, Ahmadvand and Vaezi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Sharma A, Bhatt NS, St Martin A, Abid MB, Bloomquist J, Chemaly RF, et al. Clinical characteristics and outcomes of COVID-19 in hematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol (2021) 8(3):e185–93. doi: 10.1016/s2352-3026(20)30429-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

