Four-Component Recombinant Protein-Based Vaccine Effectiveness Against Serogroup B Meningococcal Disease in Italy
- PMID: 37594762
- PMCID: PMC10439479
- DOI: 10.1001/jamanetworkopen.2023.29678
Four-Component Recombinant Protein-Based Vaccine Effectiveness Against Serogroup B Meningococcal Disease in Italy
Abstract
Importance: Population-based data on the 4-component recombinant protein-based (4CMenB) vaccine effectiveness and reduction in incidence rate ratios (IRRs) are continuously needed to assess vaccine performance in the prevention of serogroup B invasive meningococcal disease (IMD).
Objective: To assess the effectiveness and reduction in IRRs associated with the 4CMenB vaccine in the pediatric population in 6 regions in Italy.
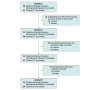
Design, setting, and participants: This retrospective cohort screening study and case-control study included data from children aged younger than 6 years in 6 highly populated Italian regions from January 1, 2006, to January 1, 2020. Participants included children younger than 6 years diagnosed with serogroup B IMD without predisposing factors. Data were collected from regional surveillance and vaccination registries and were analyzed from September 2021 to January 2022.
Exposures: Routine 4CMenB vaccination, per regional vaccination programs.
Main outcomes and measures: The main outcome was the effectiveness of the 4CMenB vaccine in the prevention of serogroup B IMD in the population of children aged younger than 6 years in 6 Italian regions. The percentages of vaccine effectiveness (VE) were obtained through the concomitant use of a screening method and a case-control study. Secondary outcomes were the comparison of effectiveness results obtained using the 2 different computational methods, the description of serogroup B IMD incidence rates, and reduction in IRRs before and after 4CMenB introduction, as a proxy for vaccine impact.
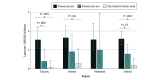
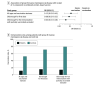
Results: The cohort screening study included a resident population of 587 561 children younger than 6 years in 3 regions with similar surveillance protocols, and the matched-case controls study assessed a resident population of 1 080 620 children younger than 6 years in 6 regions. Analyses found that 4CMenB VE in fully immunized children was 94.9% (95% CI, 83.1%-98.4%) using the screening method and 91.7% (95% CI, 24.4%-98.6%) using the case-control method. Overall reduction in IRR was 50%, reaching 70% in regions with early-start vaccination schedules. The case-control method involving 6 highly-populated Italian regions included 26 cases and 52 controls and found an estimated VE of 92.4% (95% CI, 67.6%-97.9%) in children old enough for the first vaccine dose and 95.6% (95% CI, 71.7%-99.1%) in fully immunized children. VE was more than 90% for partially immunized children. Even in regions where the first dose was administered at age 2 months, almost 20% of unvaccinated cases were among infants too young to receive the first 4CMenB dose.
Conclusions and relevance: This screening cohort study and matched case-controls study found high effectiveness of 4CMenB vaccination and greater reduction in IRR for early-start vaccination schedules in preventing invasive serogroup B meningococcal disease. The high proportion of children too young to be vaccinated among unvaccinated cases suggests that starting the vaccination even earlier may prevent more cases. Screening and case-control methods provided similar estimates of VE: either method may be used in different study settings, but concomitant use can provide more robust estimates.
Conflict of interest statement
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

