Commensal Cutibacterium acnes induce epidermal lipid synthesis important for skin barrier function
- PMID: 37595033
- PMCID: PMC10438445
- DOI: 10.1126/sciadv.adg6262
Commensal Cutibacterium acnes induce epidermal lipid synthesis important for skin barrier function
Abstract
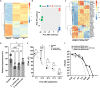
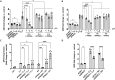
Lipid synthesis is necessary for formation of epithelial barriers and homeostasis with external microbes. An analysis of the response of human keratinocytes to several different commensal bacteria on the skin revealed that Cutibacterium acnes induced a large increase in essential lipids including triglycerides, ceramides, cholesterol, and free fatty acids. A similar response occurred in mouse epidermis and in human skin affected with acne. Further analysis showed that this increase in lipids was mediated by short-chain fatty acids produced by Cutibacterium acnes and was dependent on increased expression of several lipid synthesis genes including glycerol-3-phosphate-acyltransferase-3. Inhibition or RNA silencing of peroxisome proliferator-activated receptor-α (PPARα), but not PPARβ and PPARγ, blocked this response. The increase in keratinocyte lipid content improved innate barrier functions including antimicrobial activity, paracellular diffusion, and transepidermal water loss. These results reveal that metabolites from a common commensal bacterium have a previously unappreciated influence on the composition of epidermal lipids.
Figures



References
-
- P. M. Elias, Structure and function of the stratum corneum permeability barrier. Drug Dev. Res. 13, 97–105 (1988).
-
- G. K. Menon, G. W. Cleary, M. E. Lane, The structure and function of the stratum corneum. Int. J. Pharm. 435, 3–9 (2012). - PubMed
-
- T. A. Harris-Tryon, E. A. Grice, Microbiota and maintenance of skin barrier function. Science 376, 940–945 (2022). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

