Spic regulates one-carbon metabolism and histone methylation in ground-state pluripotency
- PMID: 37595034
- PMCID: PMC11801372
- DOI: 10.1126/sciadv.adg7997
Spic regulates one-carbon metabolism and histone methylation in ground-state pluripotency
Abstract
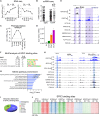
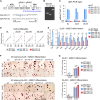
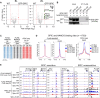
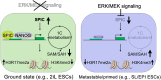
Understanding mechanisms of epigenetic regulation in embryonic stem cells (ESCs) is of fundamental importance for stem cell and developmental biology. Here, we identify Spic, a member of the ETS family of transcription factors (TFs), as a marker of ground state pluripotency. We show that Spic is rapidly induced in ground state ESCs and in response to extracellular signal-regulated kinase (ERK) inhibition. We find that SPIC binds to enhancer elements and stabilizes NANOG binding to chromatin, particularly at genes involved in choline/one-carbon (1C) metabolism such as Bhmt, Bhmt2, and Dmgdh. Gain-of-function and loss-of-function experiments revealed that Spic controls 1C metabolism and the flux of S-adenosyl methionine to S-adenosyl-L-homocysteine (SAM-to-SAH), thereby, modulating the levels of H3R17me2 and H3K4me3 histone marks in ESCs. Our findings highlight betaine-dependent 1C metabolism as a hallmark of ground state pluripotency primarily activated by SPIC. These findings underscore the role of uncharacterized auxiliary TFs in linking cellular metabolism to epigenetic regulation in ESCs.
Figures



References
-
- Y. Atlasi, H. G. Stunnenberg, The interplay of epigenetic marks during stem cell differentiation and development. Nat. Rev. Genet. 18, 643–658 (2017). - PubMed
-
- L. Weinberger, M. Ayyash, N. Novershtern, J. H. Hanna, Dynamic stem cell states: Naive to primed pluripotency in rodents and humans. Nat. Rev. Mol. Cell Biol. 17, 155–169 (2016). - PubMed
-
- Y. Atlasi, W. Megchelenbrink, T. Peng, E. Habibi, O. Joshi, S.-Y. Wang, C. Wang, C. Logie, I. Poser, H. Marks, H. G. Stunnenberg, Epigenetic modulation of a hardwired 3D chromatin landscape in two naive states of pluripotency. Nat. Cell Biol. 21, 568–578 (2019). - PubMed
-
- A. S. Bernardo, A. Jouneau, H. Marks, P. Kensche, J. Kobolak, K. Freude, V. Hall, A. Feher, Z. Polgar, C. Sartori, I. Bock, C. Louet, T. Faial, H. H. D. Kerstens, C. Bouissou, G. Parsonage, K. Mashayekhi, J. C. Smith, G. Lazzari, P. Hyttel, H. G. Stunnenberg, M. Huynen, R. A. Pedersen, A. Dinnyes, Mammalian embryo comparison identifies novel pluripotency genes associated with the naïve or primed state. Biol. Open 7, bio033282 (2018). - PMC - PubMed
-
- M. Bemark, A. Mårtensson, D. Liberg, T. Leanderson, Spi-C, a novel Ets protein that is temporally regulated during B lymphocyte development. J. Biol. Chem. 274, 10259–10267 (1999). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

