Safety and Efficacy of Baloxavir Marboxil in Influenza-infected Children 5-11 Years of Age: A Post Hoc Analysis of a Phase 3 Study
- PMID: 37595103
- PMCID: PMC10569673
- DOI: 10.1097/INF.0000000000004062
Safety and Efficacy of Baloxavir Marboxil in Influenza-infected Children 5-11 Years of Age: A Post Hoc Analysis of a Phase 3 Study
Abstract
Background: miniSTONE-2 (NCT03629184) was a global, phase 3, randomized, controlled study that investigated the safety and efficacy of single-dose baloxavir marboxil in otherwise healthy children 1-<12 years of age and showed a positive risk-benefit profile. This post hoc analysis evaluated the safety and efficacy of baloxavir versus oseltamivir in children 5-11 years old with influenza.
Methods: Children received single-dose baloxavir or twice-daily oseltamivir for 5 days. Safety was the primary objective. Efficacy and virological outcomes included time to alleviation of symptoms, duration of fever and time to cessation of viral shedding by titer. Data were summarized descriptively.
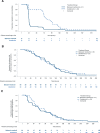
Results: Ninety-four children 5-11 years old were included (61 baloxavir and 33 oseltamivir). Baseline characteristics were similar between the groups. The incidence of adverse events was balanced and low in both treatment groups, with the most common being vomiting (baloxavir 5% vs. oseltamivir 18%), diarrhea (5% vs. 0%) and otitis media (0% vs. 5%). No serious adverse events or deaths occurred. Median (95% CI) time to alleviation of symptoms with baloxavir was 138.4 hours (116.7-163.4) versus 126.1 hours (95.9-165.7) for oseltamivir; duration of fever was comparable between groups [41.2 hours (23.5-51.4) vs. 51.3 hours (30.7-56.8), respectively]. Median time to cessation of viral shedding was shorter in the baloxavir group versus oseltamivir (1 vs. ≈3 days).
Conclusions: Safety, efficacy and virological results in children 5-11 years were similar to those from the overall study population 1-<12 years of age. Single-dose baloxavir provides an additional treatment option for pediatric patients 5-11 years old with influenza.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
This post hoc analysis was funded by F. Hoffmann LaRoche Ltd and Genentech, Inc., and this funding contributed to the trial design, data interpretation and writing of this report. The authors thank Denise Kenski, PhD, of Health Interactions, Inc, for providing editorial assistance, which was funded by F. Hoffmann LaRoche Ltd and Genentech, Inc., in accordance with Good Publication Practice (GPP2022) guidelines ( https://www.ismpp.org/gpp-2022 ). S.E.C., C.C., M.S., S.S. and S.W. are the employees of Genentech, Inc, and hold shares of Roche. L.B.M. is the employee of Roche Products Ltd and holds shares of Roche. The other authors have no conflicts of interest to disclose.
Figures

References
LinkOut - more resources
Full Text Sources

