Structural Features and Optical Properties of All-Inorganic Zero-Dimensional Halides Cs4PbBr6- xI x Obtained by Mechanochemistry
- PMID: 37595125
- PMCID: PMC10472433
- DOI: 10.1021/acsami.3c07707
Structural Features and Optical Properties of All-Inorganic Zero-Dimensional Halides Cs4PbBr6- xI x Obtained by Mechanochemistry
Abstract
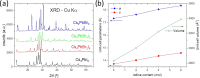
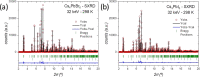
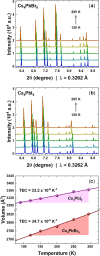
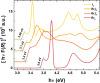
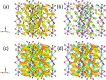
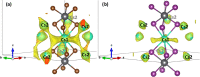
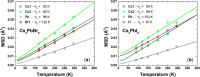
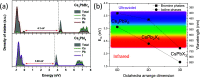
Despite the great success of hybrid CH3NH3PbI3 perovskite in photovoltaics, ascribed to its excellent optical absorption properties, its instability toward moisture is still an insurmountable drawback. All-inorganic perovskites are much less sensitive to humidity and have potential interest for solar cell applications. Alternative strategies have been developed to design novel materials with appealing properties, which include different topologies for the octahedral arrangements from three-dimensional (3D, e.g., CsPbBr3 perovskite) or two-dimensional (2D, e.g., CsPb2Br5) to zero-dimensional (0D, i.e., without connection between octahedra), as the case of Cs4PbX6 (X = Br, I) halides. The crystal structure of these materials is complex, and their thermal evolution is unexplored. In this work, we describe the synthesis of Cs4PbBr6-xIx (x = 0, 2, 4, 6) halides by mechanochemical procedures with green credentials; these specimens display excellent crystallinity enabling a detailed structural investigation from synchrotron X-ray powder diffraction (SXRD) data, essential to revisit some features in the temperature range of 90-298 K. In all this regime, the structure is defined in the trigonal R3̅c space group (#167). The presence of Cs and X vacancies suggests some ionic mobility into the crystal structure of these 0D halides. Bond valence maps (BVMs) are useful in determining isovalent surfaces for both Cs4PbBr6 and Cs4PbI6 phases, unveiling the likely ionic pathways for cesium and bromide ions and showing a full 3D connection in the bromide phase, in contrast to the iodide one. On the other hand, the evolution of the anisotropic displacement parameters is useful to evaluate the Debye temperatures, confirming that Cs atoms have more freedom to move, while Pb is more confined at its site, likely due to a higher covalency degree in Pb-X bonds than that in Cs-X bonds. Diffuse reflectance ultraviolet-visible (UV-vis) spectroscopy shows that the optical band gap can be tuned depending on iodine content (x) in the range of 3.6-3.06 eV. From density functional theory (DFT) simulations, the general trend of reducing the band gap when Br is replaced by I is well reproduced.
Keywords: Cs4PbBr6; Cs4PbI6; mechanochemical synthesis; optoelectronic properties; synchrotron X-ray diffraction; tunable band gap.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Goetzberger A.; Hoffmann V. U.. Photovoltaic Solar Energy Generation. In Springer Series in Optical Sciences; Springer: Berlin New York, 2005.
-
- Green M. A.; Emery K.; Hishikawa Y.; Warta W.; Dunlop E. D. Solar Cell Efficiency Tables (Version 45): Solar Cell Efficiency Tables. Prog. Photovoltaics: Res. Appl. 2015, 23, 1–9. 10.1002/pip.2573. - DOI
-
- Jeong J.; Kim M.; Seo J.; Lu H.; Ahlawat P.; Mishra A.; Yang Y.; Hope M. A.; Eickemeyer F. T.; Kim M.; Yoon Y. J.; Choi I. W.; Darwich B. P.; Choi S. J.; Jo Y.; Lee J. H.; Walker B.; Zakeeruddin S. M.; Emsley L.; Rothlisberger U.; Hagfeldt A.; Kim D. S.; Grätzel M.; Kim J. Y. Pseudo-Halide Anion Engineering for α-FAPbI3 Perovskite Solar Cells. Nature 2021, 592, 381–385. 10.1038/s41586-021-03406-5. - DOI - PubMed
-
- Green M. A.; Ho-Baillie A.; Snaith H. J. The Emergence of Perovskite Solar Cells. Nat. Photonics 2014, 8, 506–514. 10.1038/nphoton.2014.134. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

