The global fatty liver disease Sustainable Development Goal country score for 195 countries and territories
- PMID: 37595128
- PMCID: PMC10442089
- DOI: 10.1097/HEP.0000000000000361
The global fatty liver disease Sustainable Development Goal country score for 195 countries and territories
Abstract
Background and aims: Fatty liver disease is highly prevalent, resulting in overarching wellbeing and economic costs. Addressing it requires comprehensive and coordinated multisectoral action. We developed a fatty liver disease Sustainable Development Goal (SDG) country score to provide insights into country-level preparedness to address fatty liver disease through a whole-of-society lens.
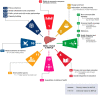
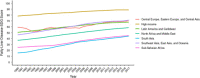
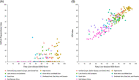
Approach and results: We developed 2 fatty liver disease-SDG score sets. The first included 6 indicators (child wasting, child overweight, noncommunicable disease mortality, a universal health coverage service coverage index, health worker density, and education attainment), covering 195 countries and territories between 1990 and 2017. The second included the aforementioned indicators plus an urban green space indicator, covering 60 countries and territories for which 2017 data were available. To develop the fatty liver disease-SDG score, indicators were categorized as "positive" or "negative" and scaled from 0 to 100. Higher scores indicate better preparedness levels. Fatty liver disease-SDG scores varied between countries and territories (n = 195), from 14.6 (95% uncertainty interval: 8.9 to 19.4) in Niger to 93.5 (91.6 to 95.3) in Japan; 18 countries and territories scored > 85. Regionally, the high-income super-region had the highest score at 88.8 (87.3 to 90.1) in 2017, whereas south Asia had the lowest score at 44.1 (42.4 to 45.8). Between 1990 and 2017, the fatty liver disease-SDG score increased in all super-regions, with the greatest increase in south Asia, but decreased in 8 countries and territories.
Conclusions: The fatty liver disease-SDG score provides a strategic advocacy tool at the national and global levels for the liver health field and noncommunicable disease advocates, highlighting the multisectoral collaborations needed to address fatty liver disease, and noncommunicable diseases overall.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
This work was supported by the EASL International Liver Foundation, which acknowledges funding from Intercept Pharmaceutics, as well as Bristol Myers Squibb and Merck Sharp and Dohme. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to the data in the study and had final responsibility for the decision to submit to publication.
Jeffrey V. Lazarus reports grants from Gilead Sciences and Roche and consulting fees and advisory arrangements from AbbVie, Gilead Sciences, and Roche, outside the submitted work. Jörn M. Schattenberg reports consulting fees from Apollo Endosurgery, AGED diagnostics, Bayer, Boehringer Ingelheim, Gilead Sciences, GSK, Intercept Pharmaceuticals, Ipsen, Inventiva Pharma, Madrigal, MSD, Northsea Therapeutics, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi, and Siemens Healthineers, speaker fees from Boehringer Ingelheim, Echosens, MedPublico GmbH, Novo Nordisk, Madrigal Pharmaceuticals, and Histoindex, and grants from Gilead Sciences, Boehringer Ingelheim, and Siemens Healthcare GmbH, outside the submitted work. Hannah Han and M. Ashworth Dirac report support for the present manuscript from the EASL International Liver Foundation. Sandra Cortés reports support from the Fondo de Financiamiento de Centros de Investigacion en Areas Prioritarias (FONDAP) (grant number 15130011), outside the submitted work. Xiaochen Dai reports support for the present manuscript from IHME/UW as a salaried employee. Temitope Cyrus Ekundayo reports grants or contracts from The African-German Network of Excellence in Science (AGNES), the Federal Ministry of Education and Research (BMBF), and the Alexander von Humboldt Foundation (AvH) for financial support, outside the submitted work. Vivek Kumar Gupta reports grants or contracts from the National Health and Medical Research Council (NHMRC), Australia, outside the submitted work. Vivian Chia-rong Hsieh reports support from the National Science and Technology Council, Taiwan for Grant # MOST 107-2314-B-039-065-MY3 and grants or contracts from the National Science and Technology Council, Taiwan for Grant # MOST 107-2314-B-039-065-MY3, outside the submitted work. Nahlah Elkudssiah Ismail reports leadership or fiduciary roles in board, society, committee, or advocacy groups, paid or unpaid, with the Malaysian Academy of Pharmacy as council member, outside the submitted work. Abel Joseph reports support from the Bill and Melinda Gates Foundation, grants or contracts from the American College of Gastroenterology for a Clinical Research Award, and support for attending meetings and/or travel from American College of Gastroenterology Annual Meeting 2022, outside the submitted work. Jacek Jerzy Jozwiak reports payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from NOVART and ADAMED, outside the submitted work. Ibraheem M. Karaye reports support for attending meetings and/or travel from Hofstra University, outside the submitted work. Yeong Yeh Lee reports grants or contracts from NAFLD-related Clinical Trial Investigator for Novo Nordisk and Boehringer Ingelheim and leadership or fiduciary roles in board, society, committee, or advocacy groups, paid or unpaid, with the Malaysian Society of Gastroenterology and Hepatology as President, outside the submitted work. Lee-Ling Lim reports grants or contracts from Boehringer Ingelheim, AstraZeneca, and Abbott Nutrition, outside the submitted work. Stefan Lorkowski acknowledges funding by the German Federal Ministry of Education and Research (nutriCARD, grant agreement number 01EA1808A), grants or contracts from Akcea Therapeutics Germany, consulting fees from Danone, Novartis Pharma, and Swedish Orphan Biovitrum (SOBI), payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Akcea Therapeutics Germany, AMARIN Germany, AMARIN Germany, AMGEN, Berlin-Chemie, Boehringer Ingelheim Pharma, Daiichi Sankyo Deutschland, Danone, Hubert Burda Media Holding, Janssen-Cilag, Lilly Deutschland, Novartis Pharma, Novo Nordisk Pharma, Roche Pharma, Sanofi-Aventis, and SYNLAB Holding Deutschland & SYNLAB Akademie, support for attending meetings and/or travel from AMGEN and Novo Nordisk Pharma., and participation on a Data Safety Monitoring Board or Advisory Board with Akcea Therapeutics Germany, AMGEN, Daiichi Sankyo Deutschland, Novartis Pharma, and Sanofi-Aventis, outside the submitted work. Chinmoy Sarkar reports grants or contracts from the US National Academy of Medicine – Hong Kong University International Fellowship in Global Health Leadership (2019-23), outside the submitted work. Pritik A. Shah reports support for the present manuscript from Bangalore Medical College and Research Institute, part of the Rajiv Gandhi University of Health Sciences, outside the submitted work. Jasvinder A. Singh reports consulting fees from Crealta/Horizon, Medisys, Fidia, PK Med, Two labs Inc., Adept Field Solutions, Clinical Care options, Clearview healthcare partners, Putnam associates, Focus forward, Navigant consulting, Spherix, MedIQ, Jupiter Life Science, UBM LLC, Trio Health, Medscape, WebMD, Practice Point communications, and the National Institutes of Health and the American College of Rheumatology, payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Simply Speaking as a member of the speakers' bureau, support for attending meetings and/or travel from the steering committee of OMERACT, participation on a Data Safety Monitoring Board or Advisory Board with FDA Arthritis Advisory Committee, leadership or fiduciary roles in board, society, committee, or advocacy groups, paid or unpaid, with OMERACT as a steering committee member, the Veterans Affairs Rheumatology Field Advisory Committee as Chair, and UAB Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis as editor and Director, stock or stock options in TPT Global Tech, Vaxart pharmaceuticals, Atyu biopharma, Adaptimmune Therapeutics, GeoVax Labs, Pieris Pharmaceuticals, Enzolytics Inc., Seres Therapeutics, Tonix Pharmaceuticals, and Charlotte’s Web Holdings, Inc, and previously held stock options in Amarin, Viking, and Moderna pharmaceuticals, outside the submitted work. The remaining authors have no conflicts to report.
Figures


References
-
- Lazarus JV, Mark HE, Villota-Rivas M, Palayew A, Carrieri P, Colombo M, et al. The global NAFLD policy review and preparedness index: Are countries ready to address this silent public health challenge? J Hepatol. 2022;76:771–80. - PubMed
-
- Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–61. - PubMed
-
- Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–93. - PubMed

