BRAF V600E is associated with higher incidence of second cancers in adults with Langerhans cell histiocytosis
- PMID: 37595284
- PMCID: PMC10797504
- DOI: 10.1182/blood.2023021212
BRAF V600E is associated with higher incidence of second cancers in adults with Langerhans cell histiocytosis
Abstract
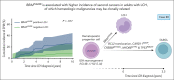
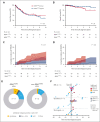
In this retrospective study, BRAF mutation status did not correlate with disease extent or (event-free) survival in 156 adults with Langerhans cell histiocytosis. BRAFV600E was associated with an increased incidence of second malignancies, often comprising hematological cancers, which may be clonally related.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: G.G. served on an advisory board for Opna Bio LLC, received royalties from UpToDate, and is a consultant to 2nd.MD. W.O.T. has received research funding from Mallinckrodt Inc, the Center for Multiple Sclerosis and Autoimmune Neurology at Mayo Clinic, and the National Institutes of Health; speaking fees from DKBMed, NeurologyLive, and the American Association for Clinical Chemistry; and publishing royalties from the publication of
Figures

References
-
- Goyal G, Tazi A, Go RS, et al. International expert consensus recommendations for the diagnosis and treatment of Langerhans cell histiocytosis in adults. Blood. 2022;139(17):2601–2621. - PubMed
-
- Allen CE, Beverley PCL, Collin M, et al. The coming of age of Langerhans cell histiocytosis. Nat Immunol. 2020;21(1):1–7. - PubMed
-
- Xiao Y, van Halteren AGS, Lei X, et al. Bone marrow–derived myeloid progenitors as driver mutation carriers in high- and low-risk Langerhans cell histiocytosis. Blood. 2020;136(19):2188–2199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

