Embedding research study recruitment within the patient portal preCheck-in
- PMID: 37595575
- PMCID: PMC10654868
- DOI: 10.1093/jamia/ocad164
Embedding research study recruitment within the patient portal preCheck-in
Abstract
Objective: Patient portals are increasingly used to recruit patients in research studies, but communication response rates remain low without tactics such as financial incentives or manual outreach. We evaluated a new method of study enrollment by embedding a study information sheet and HIPAA authorization form (HAF) into the patient portal preCheck-in (where patients report basic information like allergies).
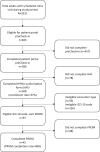
Materials and methods: Eligible patients who enrolled received an after-visit patient-reported outcomes survey through the patient portal. No additional recruitment/messaging efforts were made.
Results: A total of 386 of 843 patients completed preCheck-in, 308 of whom signed the HAF and enrolled in the study (37% enrollment rate). Of 93 patients who were eligible to receive the after-visit survey, 45 completed it (48% completion rate).
Conclusion: Enrollment and survey completion rates were higher than what is typically seen with recruitment by patient portal messaging, suggesting that preCheck-in recruitment can enhance research study recruitment and warrants further investigation.
Keywords: HIPAA authorization form; patient portal; preCheck-in; research study recruitment; survey.
© The Author(s) 2023. Published by Oxford University Press on behalf of the American Medical Informatics Association. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
None declared.
Figures


Similar articles
-
Health Insurance Portability and Accountability Act (HIPAA) authorization and survey nonresponse bias.Med Care. 2011 Apr;49(4):365-70. doi: 10.1097/MLR.0b013e318202ada0. Med Care. 2011. PMID: 21368682 Free PMC article. Clinical Trial.
-
The HIPAA authorization form and effects on survey response rates, nonresponse bias, and data quality: a randomized community study.Med Care. 2007 Oct;45(10):959-65. doi: 10.1097/MLR.0b013e31805468b0. Med Care. 2007. PMID: 17890993 Clinical Trial.
-
Use of electronic recruitment methods in a clinical trial of adults with gout.Clin Trials. 2021 Feb;18(1):92-103. doi: 10.1177/1740774520956969. Epub 2020 Sep 15. Clin Trials. 2021. PMID: 32933342 Free PMC article.
-
Current state of radiology report release in electronic patient portals.Clin Imaging. 2021 Jun;74:22-26. doi: 10.1016/j.clinimag.2020.12.020. Epub 2021 Jan 5. Clin Imaging. 2021. PMID: 33429142 Review.
-
Quantifying Patient Portal Use: Systematic Review of Utilization Metrics.J Med Internet Res. 2021 Feb 25;23(2):e23493. doi: 10.2196/23493. J Med Internet Res. 2021. PMID: 33629962 Free PMC article.

