Neoadjuvant immune checkpoint blockade: A window of opportunity to advance cancer immunotherapy
- PMID: 37595586
- PMCID: PMC10548441
- DOI: 10.1016/j.ccell.2023.07.011
Neoadjuvant immune checkpoint blockade: A window of opportunity to advance cancer immunotherapy
Abstract
Among new treatment approaches for patients with cancer, few have accelerated as quickly as neoadjuvant immune checkpoint blockade (ICB). Neoadjuvant cancer therapy is administered before curative-intent surgery in treatment-naïve patients. Conventional neoadjuvant chemotherapy and radiotherapy are primarily intended to reduce tumor size, improving surgical resectability. However, recent scientific evidence outlined here suggests that neoadjuvant immunotherapy can expand and transcriptionally modify tumor-specific T cell clones to enhance both intratumoral and systemic anti-tumor immunity. It further offers a unique "window of opportunity" to explore mechanisms and identify novel biomarkers of ICB response and resistance, opening possibilities for refining long-term clinical outcome predictions and developing new, more highly effective ICB combination therapies. Here, we examine advances in clinical and scientific knowledge gleaned from studies in select cancers and describe emerging key principles relevant to neoadjuvant ICB across many cancer types.
Keywords: anti-PD-1; anti-PD-L1; biomarker; cancer immunotherapy; clinical trial; immune checkpoint blockade; multi-omics; neoadjuvant.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests SLT and DMP receive consulting fees from Bristol Myers Squibb, Compugen, Dragonfly Therapeutics, Janssen Pharmaceuticals, PathAI, Regeneron, and Tizona LLC; receive research grants from Bristol Myers Squibb and Compugen; have stock options or stock in Dragonfly Therapeutics and Tizona LLC; and have patents related to T cell regulatory molecules including LAG-3, and the treatment of MSI-high cancers with anti-PD-1. PMF receives consulting fees from Amgen, AstraZeneca, BMS, Daiichi, Flame, Fosun, F-Star, G1, Genentech, Janssen, Iteos, Merck, Sanofi, Novartis, Regeneron, Surface, Synthekine, Tavotek, Teva; receives research grants from AstraZeneca, BMS, BioNTech, Novartis, and Regeneron; and has a patent related to the use of persistent mutation burden to predict benefit from immunotherapy in solid tumors. LAE is a current employee of Ankyra Therapeutics, with potential for future stock options; is the current President for the Society for Immunotherapy of Cancer; has received research funding to the institution for clinical research work sponsored by Abbvie, AstraZeneca, Bristol Myers Squibb, Compugen, CytomX, EMD Serono, Roche/Genentech, Immune Onc, Merck, Next Cure, Silverback Therapeutics, Takeda, and Tempest; acknowledges a consulting/advisory activity for AstraZeneca, Chugai, CytomX, Roche/Genentech, Gilead, GPCR, Immune Onc, Immutep, Mersana, and Shionogi; acknowledges Roche/Genentech for medical writing support; and has the potential for future stock options from Molecuvax. MY receives consulting fees Genentech/Roche, Exelixis, Eisai, AstraZeneca, Replimune, and Hepion; receives research grants from Bristol Myers Squibb, Incyte, and Genentech/Roche; and has equity interest in Adventris Pharmaceuticals. KNS has received honoraria/consulting fees from Adaptive Biotechnologies; receives research funding from BMS, Abbvie, AstraZeneca, and Enara; holds founder’s equity in ManaT Bio, Inc.; and has filed for patent protection related to the MANAFEST technology and T cell receptors specific for neoantigens derived from recurrent mutant oncogenes.
Figures
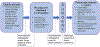


References
-
- Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, et al. (2022). Event-free survival with pembrolizumab in early triple-negative breast cancer. N. Engl. J. Med 386, 556–567. - PubMed
-
- Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D’Amico TA, et al. (2022). Non-small cell lung cancer, version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw 20, 497–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

