Macrophage-Targeted Lipid Nanoparticle Delivery of microRNA-146a to Mitigate Hemorrhagic Shock-Induced Acute Respiratory Distress Syndrome
- PMID: 37595605
- PMCID: PMC10754353
- DOI: 10.1021/acsnano.3c01814
Macrophage-Targeted Lipid Nanoparticle Delivery of microRNA-146a to Mitigate Hemorrhagic Shock-Induced Acute Respiratory Distress Syndrome
Abstract
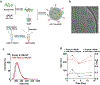
The pro-inflammatory response of alveolar macrophages to injurious physical forces during mechanical ventilation is regulated by the anti-inflammatory microRNA, miR-146a. Increasing miR-146a expression to supraphysiologic levels using untargeted lipid nanoparticles reduces ventilator-induced lung injury but requires a high initial dose of miR-146a making it less clinically applicable. In this study, we developed mannosylated lipid nanoparticles that can effectively mitigate lung injury at the initiation of mechanical ventilation with lower doses of miR-146a. We used a physiologically relevant humanized in vitro coculture system to evaluate the cell-specific targeting efficiency of the mannosylated lipid nanoparticle. We discovered that mannosylated lipid nanoparticles preferentially deliver miR-146a to alveolar macrophages and reduce force-induced inflammation in vitro. Our in vivo study using a clinically relevant mouse model of hemorrhagic shock-induced acute respiratory distress syndrome demonstrated that delivery of a low dose of miR-146a (0.1 nmol) using mannosylated lipid nanoparticles dramatically increases miR-146a levels in mouse alveolar macrophages and decreases lung inflammation. These data suggest that mannosylated lipid nanoparticles may have the therapeutic potential to mitigate lung injury during mechanical ventilation.
Keywords: Mannosylated lipid nanoparticle; acute respiratory distress syndrome; alveolar macrophages; inflammation; microRNA.
Conflict of interest statement
The authors declare no competing interests.
Figures
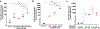
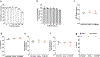
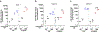


Update of
-
Macrophage-targeted lipid nanoparticle delivery of microRNA-146a to mitigate hemorrhagic shock-induced acute respiratory distress syndrome.bioRxiv [Preprint]. 2023 Feb 19:2023.02.17.529007. doi: 10.1101/2023.02.17.529007. bioRxiv. 2023. Update in: ACS Nano. 2023 Sep 12;17(17):16539-16552. doi: 10.1021/acsnano.3c01814. PMID: 36824913 Free PMC article. Updated. Preprint.
References
-
- Lee H; Fei Q; Streicher A; Zhang W; Isabelle C; Patel P; Lam HC; Arciniegas-Rubio A; Pinilla-Vera M; Amador-Munoz DP; Barragan-Bradford D; Higuera-Moreno A; Putman RK; Sholl LM; Henske EP; Bobba CM; Higuita-Castro N; Shalosky EM; Hite RD; Christman JW; Ghadiali SN; Baron RM; Englert JA, mTORC1 is a mechanosensor that regulates surfactant function and lung compliance during ventilator-induced lung injury. JCI Insight 2021, 6 (14). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

