Metabolic changes and propensity for inflammation, fibrosis, and cancer in livers of mice lacking lysosomal acid lipase
- PMID: 37595802
- PMCID: PMC10482749
- DOI: 10.1016/j.jlr.2023.100427
Metabolic changes and propensity for inflammation, fibrosis, and cancer in livers of mice lacking lysosomal acid lipase
Abstract
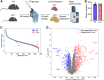
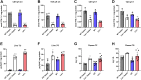
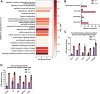
Lysosomal acid lipase (LAL) is the sole lysosomal enzyme responsible for the degradation of cholesteryl esters and triacylglycerols at acidic pH. Impaired LAL activity leads to LAL deficiency (LAL-D), a severe and fatal disease characterized by ectopic lysosomal lipid accumulation. Reduced LAL activity also contributes to the development and progression of non-alcoholic fatty liver disease (NAFLD). To advance our understanding of LAL-related liver pathologies, we performed comprehensive proteomic profiling of livers from mice with systemic genetic loss of LAL (Lal-/-) and from mice with hepatocyte-specific LAL-D (hepLal-/-). Lal-/- mice exhibited drastic proteome alterations, including dysregulation of multiple proteins related to metabolism, inflammation, liver fibrosis, and cancer. Global loss of LAL activity impaired both acidic and neutral lipase activities and resulted in hepatic lipid accumulation, indicating a complete metabolic shift in Lal-/- livers. Hepatic inflammation and immune cell infiltration were evident, with numerous upregulated inflammation-related gene ontology biological process terms. In contrast, both young and mature hepLal-/- mice displayed only minor changes in the liver proteome, suggesting that loss of LAL solely in hepatocytes does not phenocopy metabolic alterations observed in mice globally lacking LAL. These findings provide valuable insights into the mechanisms underlying liver dysfunction in LAL-D and may help in understanding why decreased LAL activity contributes to NAFLD. Our study highlights the importance of LAL in maintaining liver homeostasis and demonstrates the drastic consequences of its global deficiency on the liver proteome and liver function.
Keywords: cholesterol; cholesterol ester storage disease; lipase/lysosomal; lipid metabolism; lipids; liver; lysosomal storage disorder; non-alcoholic fatty liver disease; proteomics.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Comment in
-
Dissecting cell type-specific impact in lysosomal acid lipase deficiency-associated disorders.J Lipid Res. 2023 Dec;64(12):100474. doi: 10.1016/j.jlr.2023.100474. Epub 2023 Nov 14. J Lipid Res. 2023. PMID: 37972729 Free PMC article. No abstract available.
References
-
- Goldstein J.L., Dana S.E., Faust J.R., Beaudet A.L., Brown M.S. Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein. Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease. J. Biol. Chem. 1975;250:8487–8495. - PubMed
-
- Bernstein D.L., Hulkova H., Bialer M.G., Desnick R.J. Cholesteryl ester storage disease: review of the findings in 135 reported patients with an underdiagnosed disease. J. Hepatol. 2013;58:1230–1243. - PubMed
-
- Carter A., Brackley S.M., Gao J., Mann J.P. The global prevalence and genetic spectrum of lysosomal acid lipase deficiency: a rare condition that mimics NAFLD. J. Hepatol. 2019;70:142–150. - PubMed
-
- Reiner Z., Guardamagna O., Nair D., Soran H., Hovingh K., Bertolini S., et al. Lysosomal acid lipase deficiency--an under-recognized cause of dyslipidaemia and liver dysfunction. Atherosclerosis. 2014;235:21–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

