Mapping Essential Tremor to a Common Brain Network Using Functional Connectivity Analysis
- PMID: 37596042
- PMCID: PMC10585696
- DOI: 10.1212/WNL.0000000000207701
Mapping Essential Tremor to a Common Brain Network Using Functional Connectivity Analysis
Erratum in
-
Mapping Essential Tremor to a Common Brain Network Using Functional Connectivity Analysis.Neurology. 2024 Feb 13;102(3):e208094. doi: 10.1212/WNL.0000000000208094. Epub 2023 Dec 28. Neurology. 2024. PMID: 38165307 Free PMC article.
Abstract
Background and objectives: The cerebello-thalamo-cortical circuit plays a critical role in essential tremor (ET). However, abnormalities have been reported in multiple brain regions outside this circuit, leading to inconsistent characterization of ET pathophysiology. Here, we test whether these mixed findings in ET localize to a common functional network and whether this network has therapeutic relevance.
Methods: We conducted a systematic literature search to identify studies reporting structural or metabolic brain abnormalities in ET. We then used 'coordinate network mapping,' which leverages a normative connectome (n = 1,000) of resting-state fMRI data to identify regions commonly connected to findings across all studies. To assess whether these regions may be relevant for the treatment of ET, we compared our network with a therapeutic network derived from lesions that relieved ET. Finally, we investigated whether the functional connectivity of this ET symptom network is abnormal in an independent cohort of patients with ET as compared with healthy controls.
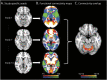
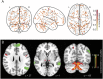
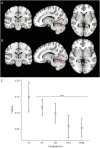
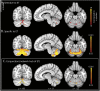
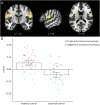
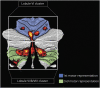
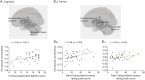
Results: Structural and metabolic brain abnormalities in ET were located in heterogeneous regions throughout the brain. However, these coordinates were connected to a common functional brain network, including the cerebellum, thalamus, motor cortex, precuneus, inferior parietal lobe, and insula. The cerebellum was identified as the hub of this network because it was the only brain region that was both functionally connected to the findings of over 90% of studies and significantly different in connectivity compared with a control data set of other movement disorders. This network was strikingly similar to the therapeutic network derived from lesions improving ET, with key regions aligning in the thalamus and cerebellum. Furthermore, positive functional connectivity between the cerebellar network hub and the sensorimotor cortices was significantly reduced in patients with ET compared with healthy controls, and connectivity within this network was correlated with tremor severity and cognitive functioning.
Discussion: These findings suggest that the cerebellum is the central hub of a network commonly connected to structural and metabolic abnormalities in ET. This network may have therapeutic utility in refining and informing new targets for neuromodulation of ET.
© 2023 American Academy of Neurology.
Conflict of interest statement
E.F.P. Younger, E.G. Ellis and N. Parsons are funded by the Deakin University Postgraduate Research Scholarship; P. Pantano has received funding for travel from Novartis, Genzyme, and Bracco and a speaking honorarium from Biogen, research support from Italian Ministry of Foreign Affairs and Fondazione Italiana Sclerosi Multipla; K. Caeyenberghs is supported by a Veski Fellowship, the Victorian Near-miss Award Pilot is administered by Veski for the Victorian Health and Medical Research Workforce Project on behalf of the Victorian Government and the Association of Australian Medical Research Institutes, funding for the Pilot has been provided by the Victorian Department of Jobs, Precincts and Regions; J. Benito-Leon is supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422), the European Commission (grant ICT-2011-287739, NeuroTREMOR), the Ministry of Economy and Competitiveness (grant RTC-2015-3967-1, NetMD—platform for the tracking of movement disorder), and the Spanish Health Research Agency (grant FIS PI12/01602 and grant FIS PI16/00451); J.P. Romero was funded by the Spanish Ministry of Science and Innovation grant (PID2020-113222RB- C21/AEI/10.13039/501100011033); J. Joutsa has received research grants from the Finnish Medical Foundation, Instrumentarium Research Foundation, Sigrid Juselius Foundation, Finnish Foundation for Alcohol Studies, University of Turku (private donation, Sigrid Juselius), Turku University Hospital (ERVA funds), and conference travel support from Abbvie and Abbott, and lecturer honoraria from Lundbeck; D.T. Corp and S. Tommasin report no disclosures relevant to the manuscript. Go to
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
