Excessive Thyroid Hormone Signaling Induces Photoreceptor Degeneration in Mice
- PMID: 37596046
- PMCID: PMC10481642
- DOI: 10.1523/ENEURO.0058-23.2023
Excessive Thyroid Hormone Signaling Induces Photoreceptor Degeneration in Mice
Abstract
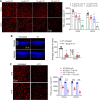
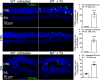
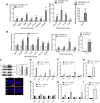
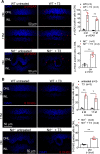
Rod and cone photoreceptors degenerate in inherited and age-related retinal degenerative diseases, ultimately leading to loss of vision. Thyroid hormone (TH) signaling regulates cell proliferation, differentiation, and metabolism. Recent studies have shown a link between TH signaling and retinal degeneration. This work investigates the effects of excessive TH signaling on photoreceptor function and survival in mice. C57BL/6, Thra1 -/-, Thrb2 -/-, Thrb -/-, and the cone dominant Nrl -/- mice received triiodothyronine (T3) treatment (5-20 μg/ml in drinking water) for 30 d, followed by evaluations of retinal function, photoreceptor survival/death, and retinal stress/damage. Treatment with T3 reduced light responses of rods and cones by 50-60%, compared with untreated controls. Outer nuclear layer thickness and cone density were reduced by ∼18% and 75%, respectively, after T3 treatment. Retinal sections prepared from T3-treated mice showed significantly increased numbers of TUNEL-positive, p-γH2AX-positive, and 8-OHdG-positive cells, and activation of Müller glial cells. Gene expression analysis revealed upregulation of the genes involved in oxidative stress, necroptosis, and inflammation after T3 treatment. Deletion of Thra1 prevented T3-induced degeneration of rods but not cones, whereas deletion of Thrb2 preserved both rods and cones. Treatment with an antioxidant partially preserved photoreceptors and reduced retinal stress responses. This study demonstrates that excessive TH signaling induces oxidative stress/damage and necroptosis, induces photoreceptor degeneration, and impairs retinal function. The findings provide insights into the role of TH signaling in retinal degeneration and support the view of targeting TH signaling for photoreceptor protection.
Keywords: cone; mice; photoreceptor; retina; rod; thyroid hormone.
Copyright © 2023 Ma et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Abdelkader M, Abass N (2019) The relation between age related macular degeneration and thyroid disorders. Int J Ophthalmol Vis Sci 4:101–105. 10.11648/j.ijovs.20190404.18 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
