Phase 2 Trial of Atezolizumab in Bacillus Calmette-Guérin-unresponsive High-risk Non-muscle-invasive Bladder Cancer: SWOG S1605
- PMID: 37596191
- PMCID: PMC10869634
- DOI: 10.1016/j.eururo.2023.08.004
Phase 2 Trial of Atezolizumab in Bacillus Calmette-Guérin-unresponsive High-risk Non-muscle-invasive Bladder Cancer: SWOG S1605
Abstract
Background: Although radical cystectomy (RC) is the standard of care for patients with bacillus Calmette-Guérin (BCG)-unresponsive high-risk non-muscle-invasive bladder cancer (NMIBC), many patients are ineligible for surgery or elect bladder preservation.
Objective: To evaluate the efficacy and safety of atezolizumab in BCG-unresponsive high-risk NMIBC.
Design, setting, and participants: This was a single-arm phase 2 trial in patients with BCG-unresponsive high-risk NMIBC who were ineligible for or declined RC.
Intervention: Intravenous atezolizumab every 3 wk for 1 yr.
Outcome measurements and statistical analysis: The primary endpoint was the pathological complete response (CR) rate for patients with carcinoma in situ (CIS) determined via mandatory biopsy at 6 mo. Event-free survival (EFS) at 18 mo for patients with non-CIS tumors and treatment-related adverse events (TRAEs) were key secondary endpoints.
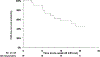
Results and limitations: Of 172 patients enrolled in the trial, 166 received at least one dose of atezolizumab (safety analysis) and 129 were eligible (efficacy analysis). Of the 74 patients with CIS, 20 (27%) experienced a CR at 6 mo. The median duration of response was 17 mo, and 56% (95% confidence interval [CI] 34-77%) of the responses were durable to at least 12 mo. The 18-mo actuarial EFS rate among 55 patients with Ta/T1 disease was 49% (90% CI 38-60%). Twelve of 129 eligible patients experienced progression to muscle-invasive or metastatic disease. Grade 3-5 TRAEs occurred in 26 patients (16%), including three treatment-related deaths. The study was limited by the small sample size and a high rate of patient ineligibility.
Conclusions: The efficacy of atezolizumab observed among patients with BCG-unresponsive NMIBC is similar to results from similar trials with other agents, but did not meet the prespecified efficacy threshold. Modest efficacy needs to be balanced with a significant rate of TRAEs and the risk of disease progression when considering systemic immunotherapy in early-stage bladder cancer.
Patient summary: We tested intravenous immunotherapy (atezolizumab) in patients with high-risk non-muscle-invasive bladder cancer that recurred after BCG (bacillus Calmette-Guérin) treatment. Although we found similar outcomes to previous trials, the benefit of this therapy is modest and needs to be carefully balanced with the significant risk of side effects. This trial is registered on ClinicalTrials.gov as NCT02844816.
Keywords: Bacillus Calmette-Guérin–unresponsive; Bladder cancer; Immune checkpoint inhibitor.
Copyright © 2023 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Figures

Comment in
-
Use of atezolizumab in bacillus Calmette-Guérin-unresponsive high-risk non-muscle-invasive bladder cancer.Transl Androl Urol. 2024 Jun 30;13(6):1061-1063. doi: 10.21037/tau-23-630. Epub 2024 May 24. Transl Androl Urol. 2024. PMID: 38983471 Free PMC article. No abstract available.
-
Balancing risks and benefits in the treatment of patients with Bacillus Calmette-Guerin-unresponsive high-risk non-muscle-invasive bladder cancer.Transl Androl Urol. 2025 Jan 31;14(1):1-3. doi: 10.21037/tau-24-529. Epub 2025 Jan 16. Transl Androl Urol. 2025. PMID: 39974809 Free PMC article. No abstract available.
References
-
- Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 2000;163:1124–9. - PubMed
-
- Martin-Doyle W, Leow JJ, Orsola A, Chang SL, Bellmunt J. Improving selection criteria for early cystectomy in high-grade T1 bladder cancer: a meta-analysis of 15,215 patients. J Clin Oncol 2015;33:643–50. - PubMed
-
- Cookson MS, Herr HW, Zhang ZF, Soloway S, Sogani PC, Fair WR. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol 1997;158:62–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

