Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies
- PMID: 37596267
- PMCID: PMC10439210
- DOI: 10.1038/s41392-023-01559-5
Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies
Abstract
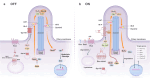
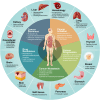
The past decade has seen significant advances in our understanding of Hedgehog (HH) signaling pathway in various biological events. HH signaling pathway exerts its biological effects through a complex signaling cascade involved with primary cilium. HH signaling pathway has important functions in embryonic development and tissue homeostasis. It plays a central role in the regulation of the proliferation and differentiation of adult stem cells. Importantly, it has become increasingly clear that HH signaling pathway is associated with increased cancer prevalence, malignant progression, poor prognosis and even increased mortality. Understanding the integrative nature of HH signaling pathway has opened up the potential for new therapeutic targets for cancer. A variety of drugs have been developed, including small molecule inhibitors, natural compounds, and long non-coding RNA (LncRNA), some of which are approved for clinical use. This review outlines recent discoveries of HH signaling in tissue homeostasis and cancer and discusses how these advances are paving the way for the development of new biologically based therapies for cancer. Furthermore, we address status quo and limitations of targeted therapies of HH signaling pathway. Insights from this review will help readers understand the function of HH signaling in homeostasis and cancer, as well as opportunities and challenges of therapeutic targets for cancer.
© 2023. West China Hospital, Sichuan University.
Conflict of interest statement
The authors declare no competing interests. C.Z., who is a member of the editorial board of Signal Transduction and Targeted Therapy, was not involved in the handling process of this manuscript.
Figures



References
-
- Ingham, P. Drosophila segment polarity mutants and the rediscovery of the hedgehog pathway genes. Curr. Top. Dev. Biol.116, 477–488 (2016). - PubMed
-
- Ingham, P. Hedgehog signaling. Curr. Top. Dev. Biol.149, 1–58 (2022). - PubMed
-
- Briscoe, J. & Thérond, P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol.14, 416–429 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

