Reactivated endogenous retroviruses promote protein aggregate spreading
- PMID: 37596282
- PMCID: PMC10439213
- DOI: 10.1038/s41467-023-40632-z
Reactivated endogenous retroviruses promote protein aggregate spreading
Abstract
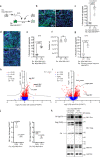
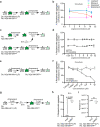
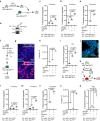
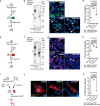
Prion-like spreading of protein misfolding is a characteristic of neurodegenerative diseases, but the exact mechanisms of intercellular protein aggregate dissemination remain unresolved. Evidence accumulates that endogenous retroviruses, remnants of viral germline infections that are normally epigenetically silenced, become upregulated in neurodegenerative diseases such as amyotrophic lateral sclerosis and tauopathies. Here we uncover that activation of endogenous retroviruses affects prion-like spreading of proteopathic seeds. We show that upregulation of endogenous retroviruses drastically increases the dissemination of protein aggregates between cells in culture, a process that can be inhibited by targeting the viral envelope protein or viral protein processing. Human endogenous retrovirus envelopes of four different clades also elevate intercellular spreading of proteopathic seeds, including pathological Tau. Our data support a role of endogenous retroviruses in protein misfolding diseases and suggest that antiviral drugs could represent promising candidates for inhibiting protein aggregate spreading.
© 2023. Springer Nature Limited.
Conflict of interest statement
S.L., S.A.M., S.F.L., P.D., and I.M.V. hold pending patent applications for “HERV inhibitors for use in treating tauopathies”: “US Patent Application No. 17/640,119 based on PCT International Application No. PCT/EP2020/074809, claiming priority to “European Application No. 19195304.1”. The remaining authors declare no competing interests.
Figures



References
-
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

