Cost effectiveness review of text messaging, smartphone application, and website interventions targeting T2DM or hypertension
- PMID: 37596488
- PMCID: PMC10439143
- DOI: 10.1038/s41746-023-00876-x
Cost effectiveness review of text messaging, smartphone application, and website interventions targeting T2DM or hypertension
Abstract
Digital health interventions have been shown to be clinically-effective for type 2 diabetes mellitus (T2DM) and hypertension prevention and treatment. This study synthesizes and compares the cost-effectiveness of text-messaging, smartphone application, and websites by searching CINAHL, Cochrane Central, Embase, Medline and PsycInfo for full economic or cost-minimisation studies of digital health interventions in adults with or at risk of T2DM and/or hypertension. Costs and health effects are synthesised narratively. Study quality appraisal using the Consensus on Health Economic Criteria (CHEC) list results in recommendations for future health economic evaluations of digital health interventions. Of 3056 records identified, 14 studies are included (7 studies applied text-messaging, 4 employed smartphone applications, and 5 used websites). Ten studies are cost-utility analyses: incremental cost-utility ratios (ICUR) vary from dominant to €75,233/quality-adjusted life year (QALY), with a median of €3840/QALY (interquartile range €16,179). One study finds no QALY difference. None of the three digital health intervention modes is associated with substantially better cost-effectiveness. Interventions are consistently cost-effective in populations with (pre)T2DM but not in populations with hypertension. Mean quality score is 63.0% (standard deviation 13.7%). Substandard application of time horizon, sensitivity analysis, and subgroup analysis next to transparency concerns (regarding competing alternatives, perspective, and costing) downgrades quality of evidence. In conclusion, smartphone application, text-messaging, and website-based interventions are cost-effective without substantial differences between the different delivery modes. Future health economic studies should increase transparency, conduct sufficient sensitivity analyses, and appraise the ICUR more critically in light of a reasoned willingness-to-pay threshold.Registration: PROSPERO (CRD42021247845).
© 2023. Springer Nature Limited.
Conflict of interest statement
L.A. received consulting fees from Mundipharma for advice on cost-effectiveness of SGLT2 inhibitors for the management of type 2 diabetes; honoraria from Boehringer Ingelheim and Mundipharma for lectures on health economic aspects of diabetes; is a member of the board of the AstraZeneca Foundation. C.d.A. and S.G. are employees of the company Meteda, manufacturer of MetaClinic, DiaWatch METEDA, MetaDieta and MyDiet. All other authors declare no competing interests.
Figures
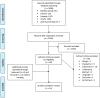

References
-
- IDF. IDF Atlas 10th edn (International Diabetes Federation, 2021).
-
- Morris DH, et al. Progression rates from HbA1c 6.0-6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia. 2013;56:1489–1493. - PubMed
-
- Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension (Dallas, Tex. : 1979) 2001;37:1053–1059. - PubMed
-
- Egan BM, Stevens-Fabry S. Prehypertension–prevalence, health risks, and management strategies. Nat. Rev. Cardiol. 2015;12:289–300. - PubMed
Publication types
Grants and funding
- 945246/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945246/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945246/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945246/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945246/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 945246/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- APP2007006/Department of Health | National Health and Medical Research Council (NHMRC)
- APP2007006/Department of Health | National Health and Medical Research Council (NHMRC)
- APP2007006/Department of Health | National Health and Medical Research Council (NHMRC)
- APP2007006/Department of Health | National Health and Medical Research Council (NHMRC)
LinkOut - more resources
Full Text Sources

