Age-adjusted high-dose chemotherapy followed by autologous stem cell transplantation or conventional chemotherapy with R-MP as first-line treatment in elderly primary CNS lymphoma patients - the randomized phase III PRIMA-CNS trial
- PMID: 37596517
- PMCID: PMC10436648
- DOI: 10.1186/s12885-023-11193-7
Age-adjusted high-dose chemotherapy followed by autologous stem cell transplantation or conventional chemotherapy with R-MP as first-line treatment in elderly primary CNS lymphoma patients - the randomized phase III PRIMA-CNS trial
Abstract
Background: Older primary central nervous system lymphoma (PCNSL) patients have an inferior prognosis compared to younger patients because available evidence on best treatment is scarce and treatment delivery is challenging due to comorbidities and reduced performance status. High-dose chemotherapy and autologous stem cell transplantation (HCT-ASCT) after high-dose methotrexate (MTX)-based immuno-chemotherapy has become an increasingly used treatment approach in eligible elderly PCNSL patients with promising feasibility and efficacy, but has not been compared with conventional chemotherapy approaches. In addition, eligibility for HCT-ASCT in elderly PCNSL is not well defined. Geriatric assessment (GA) may be helpful in selecting patients for the best individual treatment choice, but no standardized GA exists to date. A randomized controlled trial, incorporating a GA and comparing age-adapted HCT-ASCT treatment with conventional chemotherapy is needed.
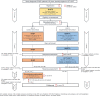
Methods: This open-label, multicenter, randomized phase III trial with two parallel arms will recruit 310 patients with newly diagnosed PCNSL > 65 years of age in 40 centers in Germany and Austria. The primary objective is to demonstrate that intensified chemotherapy followed by consolidating HCT-ASCT is superior to conventional chemotherapy with rituximab, MTX, procarbazine (R-MP) followed by maintenance with procarbazine in terms of progression free survival (PFS). Secondary endpoints include overall survival (OS), event free survival (EFS), (neuro-)toxicity and quality of life (QoL). GA will be conducted at specific time points during the course of the study. All patients will be treated with a pre-phase rituximab-MTX (R-MTX) cycle followed by re-assessment of transplant eligibility. Patients judged transplant eligible will be randomized (1:1). Patients in arm A will be treated with 3 cycles of R-MP followed by maintenance therapy with procarbazine for 6 months. Patients in arm B will be treated with 2 cycles of MARTA (R-MTX/AraC) followed by busulfan- and thiotepa-based HCT-ASCT.
Discussion: The best treatment strategy for elderly PCNSL patients remains unknown. Treatments range from palliative to curative but more toxic therapies, and there is no standardized measure to select patients for the right treatment. This randomized controlled trial will create evidence for the best treatment strategy with the focus on developing a standardized GA to help define eligibility for an intensive treatment approach.
Trial registration: German clinical trials registry DRKS00024085 registered March 29, 2023.
Keywords: Autologous stem cell transplantation (ASCT); Elderly patients; High-dose chemotherapy (HCT); Primary central nervous system lymphoma (PCNSL); Transplant eligibility.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
ES and GI received speakers’ honoraria and research funding from Riemser Pharma GmbH. GI received research funding from Roche Pharma AG and AbbVie, outside the submitted work. All other authors declare no conflict of interest.
References
-
- Schorb E, Fox CP, Kasenda B, Linton K, Martinez-Calle N, Calimeri T et al. Induction therapy with the MATRix regimen in patients with newly diagnosed primary diffuse large B-cell lymphoma of the central nervous system - an international study of feasibility and efficacy in routine clinical practice. Br J Haematol. 2020;published online ahead of print, 2020 Jan 29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical