PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer
- PMID: 37596643
- PMCID: PMC10436543
- DOI: 10.1186/s12943-023-01827-6
PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer
Abstract
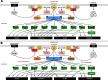
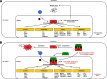
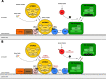
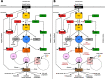
The PI3K/AKT/mTOR (PAM) signaling pathway is a highly conserved signal transduction network in eukaryotic cells that promotes cell survival, cell growth, and cell cycle progression. Growth factor signalling to transcription factors in the PAM axis is highly regulated by multiple cross-interactions with several other signaling pathways, and dysregulation of signal transduction can predispose to cancer development. The PAM axis is the most frequently activated signaling pathway in human cancer and is often implicated in resistance to anticancer therapies. Dysfunction of components of this pathway such as hyperactivity of PI3K, loss of function of PTEN, and gain-of-function of AKT, are notorious drivers of treatment resistance and disease progression in cancer. In this review we highlight the major dysregulations in the PAM signaling pathway in cancer, and discuss the results of PI3K, AKT and mTOR inhibitors as monotherapy and in co-administation with other antineoplastic agents in clinical trials as a strategy for overcoming treatment resistance. Finally, the major mechanisms of resistance to PAM signaling targeted therapies, including PAM signaling in immunology and immunotherapies are also discussed.
Keywords: AKT inhibitors; ATP-competitive mTOR inhibitors; Allosteric mTOR inhibitors; Bi-steric mTOR inhibitors; Cancer; Dual PI3K/mTOR inhibitors; Isoform-specific PI3K inhibitors; PDK1 inhibitors; PI3K/AKT/mTORC pathway; Pan PI3K inhibitors.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol. 2018;15(5):273–291. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

