Real-Space Observation of Ligand Hole State in Cubic Perovskite SrFeO3
- PMID: 37596717
- PMCID: PMC10582404
- DOI: 10.1002/advs.202302839
Real-Space Observation of Ligand Hole State in Cubic Perovskite SrFeO3
Abstract
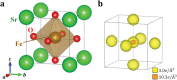
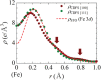
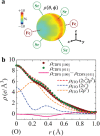
An anomalously high valence state sometimes shows up in transition-metal oxide compounds. In such systems, holes tend to occupy mainly the ligand p orbitals, giving rise to interesting physical properties such as superconductivity in cuprates and rich magnetic phases in ferrates. However, no one has ever observed the distribution of ligand holes in real space. Here, a successful observation of the spatial distribution of valence electrons in cubic perovskite SrFeO3 by high-energy X-ray diffraction experiments and precise electron density analysis using a core differential Fourier synthesis method is reported. A real-space picture of ligand holes formed by the orbital hybridization of Fe 3d and O 2p is revealed. The anomalous valence state in Fe is attributed to the considerable contribution of the ligand hole, which is related to the metallic nature and the absence of Jahn-Teller distortions in this system.
Keywords: electron orbital; ligand hole; orbital hybridization; perovskite oxide; x-ray diffraction.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Zaanen J., Sawatzky G. A., Allen J. W., Phys. Rev. Lett. 1985, 55, 418. - PubMed
-
- Eskes H., Sawatzky G. A., Phys. Rev. Lett. 1988, 61, 1415. - PubMed
-
- Eskes H., Tjeng L. H., Sawatzky G. A., Phys. Rev. B 1990, 41, 288. - PubMed
-
- Katsura H., Nagaosa N., Balatsky A. V., Phys. Rev. Lett. 2005, 95, 057205. - PubMed
-
- Mizokawa T., Namatame H., Fujimori A., Akeyama K., Kondoh H., Kuroda H., Kosugi N., Phys. Rev. Lett. 1991, 67, 1638. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
