Ketamine improves neuronal recovery following spreading depolarization in peri-infarct tissues
- PMID: 37596720
- PMCID: PMC10986311
- DOI: 10.1111/jnc.15923
Ketamine improves neuronal recovery following spreading depolarization in peri-infarct tissues
Abstract
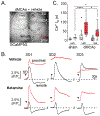
Spreading depolarization (SD) has emerged as an important contributor to the enlargement of acute brain injuries. We previously showed that the N-methyl-D-aspartate receptor antagonist ketamine was able to prevent deleterious consequences of SD in brain slices, under conditions of metabolic compromise. The current study aimed to extend these observations into an in vivo stroke model, to test whether gradients of metabolic capacity lead to differential accumulation of calcium (Ca2+) following SD. In addition, we tested whether ketamine protects vulnerable tissuewhile allowing SD to propagate through surrounding undamaged tissue. Focal lesions were generated using a distal middle cerebral artery occlusion in mice, and clusters of SD were generated at 20 min intervals with remote microinjection of potassium chloride. SDs invading peri-infarct regions had significantly different consequences, depending on the distance from the infarct core. Proximal to the lesion, Ca2+ transients were extended, as compared with responses in better-perfused tissue more remote from the lesion. Extracellular potential shifts were also longer and hyperemia responses were reduced in proximal regions following SDs. Consistent with in vitro studies, ketamine, at concentrations that did not abolish the propagation of SD, reduced the accumulation of intracellular Ca2+ in proximal regions following an SD wave. These findings suggest that deleterious consequences of SD can be targeted in vivo, without requiring outright block of SD initiation and propagation.
Keywords: NMDA receptor; calcium; excitotoxicity; spreading depression; stroke.
© 2023 International Society for Neurochemistry.
Conflict of interest statement
Conflicts of interest: None
=> if ‘none’, insert “The authors have no conflict of interest to declare.”
=> else insert info unless it is already included
Figures



Similar articles
-
Ketamine reduces deleterious consequences of spreading depolarizations.Exp Neurol. 2018 Jul;305:121-128. doi: 10.1016/j.expneurol.2018.04.007. Epub 2018 Apr 10. Exp Neurol. 2018. PMID: 29653188 Free PMC article.
-
Memantine Improves Recovery After Spreading Depolarization in Brain Slices and can be Considered for Future Clinical Trials.Neurocrit Care. 2021 Oct;35(Suppl 2):135-145. doi: 10.1007/s12028-021-01351-9. Epub 2021 Oct 17. Neurocrit Care. 2021. PMID: 34657268 Free PMC article.
-
Ketamine-induced prevention of SD-associated late infarct progression in experimental ischemia.Sci Rep. 2024 May 3;14(1):10186. doi: 10.1038/s41598-024-59835-5. Sci Rep. 2024. PMID: 38702377 Free PMC article.
-
Multifaceted roles for astrocytes in spreading depolarization: A target for limiting spreading depolarization in acute brain injury?Glia. 2016 Jan;64(1):5-20. doi: 10.1002/glia.22824. Epub 2015 Aug 24. Glia. 2016. PMID: 26301517 Free PMC article. Review.
-
Periinfarct depolarizations.Cerebrovasc Brain Metab Rev. 1996 Fall;8(3):195-208. Cerebrovasc Brain Metab Rev. 1996. PMID: 8870974 Review.
Cited by
-
Contralesional hippocampal spreading depolarization promotes functional recovery after stroke.Nat Commun. 2025 Apr 10;16(1):3428. doi: 10.1038/s41467-025-57119-8. Nat Commun. 2025. PMID: 40210646 Free PMC article.
-
Anesthetics in pathological cerebrovascular conditions.J Cereb Blood Flow Metab. 2025 Jan;45(1):32-47. doi: 10.1177/0271678X241295857. Epub 2024 Oct 25. J Cereb Blood Flow Metab. 2025. PMID: 39450477 Free PMC article. Review.
-
Synaptic Zn2+ contributes to deleterious consequences of spreading depolarizations.Neurobiol Dis. 2024 Feb;191:106407. doi: 10.1016/j.nbd.2024.106407. Epub 2024 Jan 9. Neurobiol Dis. 2024. PMID: 38199272 Free PMC article.
-
Synaptic Zn2+ contributes to deleterious consequences of spreading depolarizations.bioRxiv [Preprint]. 2023 Oct 30:2023.10.27.564408. doi: 10.1101/2023.10.27.564408. bioRxiv. 2023. Update in: Neurobiol Dis. 2024 Feb;191:106407. doi: 10.1016/j.nbd.2024.106407. PMID: 37961648 Free PMC article. Updated. Preprint.
-
Decoding ischemic stroke: Perspectives on the endoplasmic reticulum, mitochondria, and their crosstalk.Redox Biol. 2025 May;82:103622. doi: 10.1016/j.redox.2025.103622. Epub 2025 Mar 27. Redox Biol. 2025. PMID: 40188640 Free PMC article. Review.
References
-
- Abou-Chebl A, Lin R, Hussain MS, Jovin TG, Levy EI, Liebeskind DS, Yoo AJ, Hsu DP, Rymer MM, Tayal AH, Zaidat OO, Natarajan SK, Nogueira RG, Nanda A, Tian M, Hao Q, Kalia JS, Nguyen TN, Chen M and Gupta R (2010). “Conscious sedation versus general anesthesia during endovascular therapy for acute anterior circulation stroke: preliminary results from a retrospective, multicenter study.” Stroke 41(6): 1175–1179. - PubMed
-
- Busch E, Gyngell ML, Eis M, Hoehn-Berlage M and Hossmann KA (1996). “Potassium-induced cortical spreading depressions during focal cerebral ischemia in rats: contribution to lesion growth assessed by diffusion-weighted NMR and biochemical imaging.” J Cereb Blood Flow Metab 16(6): 1090–1099. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

