Establishment of a new method to isolate viable x-ray-sensitive cells from planarian by fluorescence-activated cell sorting
- PMID: 37596847
- PMCID: PMC11520976
- DOI: 10.1111/dgd.12886
Establishment of a new method to isolate viable x-ray-sensitive cells from planarian by fluorescence-activated cell sorting
Abstract
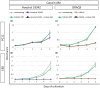
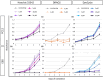
Planarians show outstanding regenerative ability due to the proliferation of neoblasts. Hence the method to isolate planarian neoblasts is important to understand the regeneration process. In our previous study, we reported a method to isolate planarian neoblasts of Dugesia japonica using fluorescence-activated cell sorting (FACS). However, we have not yet succeeded in cultivating these cells even under in vivo conditions after transplantation into x-ray-irradiated planarians. This suggests that dissociated cells might enter apoptotic or necrotic states in the process of fluorescent dye staining and sorting. Here, we developed a new method to isolate viable neoblasts, which can proliferate in the x-ray-irradiated planarians. First, the toxicity of various fluorescence dyes was investigated. All nuclear fluorescent dyes such as Hoechst 33342, DRAQ5, and DyeCycle, showed, more or less, toxicity to mammalian culture cells. In contrast, cytoplasmic fluorescent dye for live cells, calcein AM, was less toxic on these cells. Next, we stained the dissociated planarian cells with only calcein AM, and then collected the x-ray-sensitive fraction. Although the purity of neoblasts was slightly lower than that of the original staining method (ca. 97% → ca. 89%), the sorted cells could actively proliferate when they were injected into x-ray-irradiated planarians. This simple staining and sorting method will provide new opportunities to isolate viable neoblasts and understand regenerating processes.
Keywords: calcein AM; fluorescence-activated cell sorting; neoblasts; planarian; transplantation.
© 2023 The Authors. Development, Growth & Differentiation published by John Wiley & Sons Australia, Ltd on behalf of Japanese Society of Developmental Biologists.
Figures






Similar articles
-
Isolation of planarian X-ray-sensitive stem cells by fluorescence-activated cell sorting.Dev Growth Differ. 2006 Aug;48(6):371-80. doi: 10.1111/j.1440-169X.2006.00876.x. Dev Growth Differ. 2006. PMID: 16872450
-
Purification of Planarian Stem Cells Using a Draq5-Based FACS Approach.Methods Mol Biol. 2024;2805:203-212. doi: 10.1007/978-1-0716-3854-5_14. Methods Mol Biol. 2024. PMID: 39008184
-
A Subtractive FACS Method for Isolation of Planarian Stem Cells and Neural Cells.Methods Mol Biol. 2018;1774:467-478. doi: 10.1007/978-1-4939-7802-1_19. Methods Mol Biol. 2018. PMID: 29916172
-
Cellular and molecular dissection of pluripotent adult somatic stem cells in planarians.Dev Growth Differ. 2010 Jan;52(1):27-41. doi: 10.1111/j.1440-169X.2009.01155.x. Dev Growth Differ. 2010. PMID: 20078652 Review.
-
Planarians, a tale of stem cells.Cell Mol Life Sci. 2008 Jan;65(1):16-23. doi: 10.1007/s00018-007-7426-y. Cell Mol Life Sci. 2008. PMID: 18030424 Free PMC article. Review.
Cited by
-
Isolation of planarian viable cells using fluorescence-activated cell sorting for advancing single-cell transcriptome analysis.Genes Cells. 2023 Nov;28(11):800-810. doi: 10.1111/gtc.13068. Epub 2023 Sep 18. Genes Cells. 2023. PMID: 37723830 Free PMC article.
-
Advancements in Single-Cell Proteomics and Mass Spectrometry-Based Techniques for Unmasking Cellular Diversity in Triple Negative Breast Cancer.Proteomics Clin Appl. 2025 Jan;19(1):e202400101. doi: 10.1002/prca.202400101. Epub 2024 Nov 21. Proteomics Clin Appl. 2025. PMID: 39568435 Free PMC article. Review.
References
-
- Agata, K. , & Watanabe, K. (1999). Molecular and cellular aspects of planarian regeneration. Cell & Developmental Biology, 10, 377–383. - PubMed
-
- Fernandéz‐Taboada, E. , Moritz, S. , Zeuschner, D. , Stehling, M. , Schöler, H. R. , Saló, E. , & Gentile, L. (2010). Smed‐SmB, a member of the LSm protein superfamily, is essential for chromatoid body organization and planarian stem cell proliferation. Development, 137(9), 1055–1065. 10.1242/dev.051847 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

