Efficacy and safety of immune checkpoint inhibitors for advanced non-small cell lung cancer with or without PD-L1 selection: A systematic review and network meta-analysis
- PMID: 37596898
- PMCID: PMC10508436
- DOI: 10.1097/CM9.0000000000002750
Efficacy and safety of immune checkpoint inhibitors for advanced non-small cell lung cancer with or without PD-L1 selection: A systematic review and network meta-analysis
Abstract
Background: Immune checkpoint inhibitors (ICIs) are standard treatments for advanced non-small cell lung cancer (NSCLC); however, evidence regarding their relative efficacy and safety is lacking. This study compared the efficacy and safety of all currently available ICI treatments in patients with advanced NSCLC to identify optimal treatment regimens.
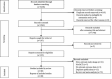
Methods: PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase databases were systematically searched for randomized controlled trials (RCTs) published up to August 8, 2022. The primary outcomes were overall survival (OS) and progression-free survival (PFS). Secondary outcomes included objective response rate (ORR) and adverse events (AEs).
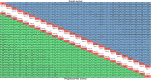
Results: Forty RCTs involving 22,526 patients were selected, and a total of 26 treatment regimens were identified. Treatment with anti-programmed cell death protein-1 (anti-PD-1) provided superior OS compared with anti-programmed death ligand 1 (anti-PD-L1) treatment. ICIs plus platinum-based chemotherapy (PBC) were superior to ICIs treatment alone, although the addition of PBC increased treatment toxicity. Cemiplimab ranked first for OS and lowest for any-grade AEs in advanced NSCLC patients without PD-L1 selection. Regarding grade ≥3 AEs, the toxicity of ICI monotherapy or ICI-ICI combination was consistently lower than that of the other treatments. For patients without PD-L1 selection, cemiplimab showed the best OS, pembrolizumab plus docetaxel (Pem-DXT) showed the best PFS, and atezolizumab plus bevacizumab and PBC (Atezo-Beva-PBC) showed the best ORR. Pembrolizumab plus PBC and Atezo-Beva-PBC were the most likely optimal treatments for OS and PFS in patients with PD-L1 expression <1%, respectively. In patients with PD-L1 expression ≥1%, treatment regimens containing anti-PD-1 provided superior OS benefits compared with those of anti-PD-L1 treatment, and sintilimab plus PBC (Sint-PBC) provided the best OS benefit; as for PFS, ICI plus PBC consistently showed greater PFS benefits than ICI or PBC alone. For patients with anti-PD-L1 expression of 1-49%, camrelizumab plus PBC provided the best benefit for OS and PFS among included treatment. Durvalumab-tremelimumab-PBC and Atezo-Beva-PBC respectively presented the highest OS and PFS for patients with PD-L1 expression ≥50%. Moreover, cemiplimab and Atezo-Beva-PBC yielded the best OS and PFS benefits as first-line treatments for patients with advanced NSCLC, respectively.
Conclusions: Although ICI plus PBC likely resulted in superior survival outcomes compared to ICI treatment alone, it did increase toxicity. Cemiplimab presented a well-balanced efficacy and safety profile in advanced NSCLC treatment. Our findings with the current ICIs comparisons will aid future trials for cancer immunotherapy.
Registration: PROSPERO, https://www.crd.york.ac.uk/PROSPERO/ , CRD42022323879.
Copyright © 2023 The Chinese Medical Association, produced by Wolters Kluwer, Inc. under the CC-BY-NC-ND license.
Conflict of interest statement
None.
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70: 7–30. doi: 10.3322/caac.21590. - PubMed
-
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553: 446–454. doi: 10.1038/nature25183. - PubMed
-
- National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology: Non-small cell lung cancer, version 4. Available from: https://www.nccn.org/guidelines/. [Last accessed on August 23, 2022].
-
- Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: A review. JAMA 2019;322: 764–774. doi: 10.1001/jama.2019.11058. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

