Using Taxol-sensitized budding yeast to investigate the effect of microtubule stabilization on anaphase onset
- PMID: 37597189
- PMCID: PMC10469069
- DOI: 10.1016/j.xpro.2023.102522
Using Taxol-sensitized budding yeast to investigate the effect of microtubule stabilization on anaphase onset
Abstract
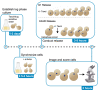
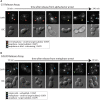
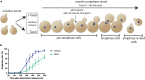
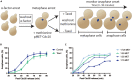
The microtubule (MT)-stabilizing drug Taxol (paclitaxel) is a commonly used tool to investigate MT dynamics and MT-dependent processes. Here, we present a protocol for using Taxol-sensitized budding yeast to investigate the effect of microtubule stabilization on anaphase onset. We describe steps for establishing a log phase culture, synchronizing cells in G1, arresting in metaphase, and releasing cells into Taxol. We then detail procedures for imaging and scoring anaphase onset. This protocol facilitates maintenance and reproducibility in testing drug-sensitized and Taxol-sensitized yeast strains. For complete details on the use and execution of this protocol, please refer to Proudfoot et al.1.
Keywords: Cell Biology; Cell-based Assays; Genetics; Model Organisms.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Proudfoot K.G., Anderson S.J., Dave S., Bunning A.R., Sinha Roy P., Bera A., Gupta M.L., Jr. Checkpoint proteins Bub1 and Bub3 delay anaphase onset in response to low tension independent of microtubule-kinetochore detachment. Cell Rep. 2019;27:416–428.e4. doi: 10.1016/j.celrep.2019.03.027. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

