The mitochondria-targeted sulfide delivery molecule attenuates drugs-induced gastropathy. Involvement of heme oxygenase pathway
- PMID: 37597422
- PMCID: PMC10458696
- DOI: 10.1016/j.redox.2023.102847
The mitochondria-targeted sulfide delivery molecule attenuates drugs-induced gastropathy. Involvement of heme oxygenase pathway
Abstract
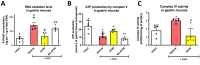
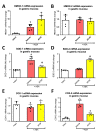
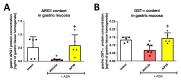
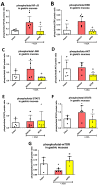
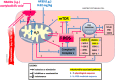
Hydrogen sulfide (H2S) signaling and H2S-prodrugs maintain redox balance in gastrointestinal (GI) tract. Predominant effect of any H2S-donor is mitochondrial. Non-targeted H2S-moieties were shown to decrease the non-steroidal anti-inflammatory drugs (NSAIDs)-induced gastrotoxicity but in high doses. However, direct, controlled delivery of H2S to gastric mucosal mitochondria as a molecular target improving NSAIDs-pharmacology remains overlooked. Thus, we treated Wistar rats, i.g. with vehicle, mitochondria-targeted H2S-releasing AP39 (0.004-0.5 mg/kg), AP219 (0.02 mg/kg) as structural control without H2S-releasing ability, or AP39 + SnPP (10 mg/kg) as a heme oxygenase (HMOX) inhibitor. Next, animals were administered i.g. with acetylsalicylic acid (ASA, 125 mg/kg) as NSAIDs representative or comparatively with 75% ethanol to induce translational hemorrhagic or necrotic gastric lesions, that were assessed micro-/macroscopically. Activity of mitochondrial complex IV/V, and DNA oxidation were assessed biochemically. Gastric mucosal/serum content of IL-1β, IL-10, TNF-α, TGF-β1/2, ARG1, GST-α, or phosphorylation of mTOR, NF-κB, ERK, Akt, JNK, STAT3/5 were evaluated by microbeads-fluorescent xMAP®-assay; gastric mucosal mRNA level of HMOX-1/2, COX-1/2, SOD-1/2 by real-time PCR. AP39 (but not AP219) dose-dependently (0.02 and 0.1 mg/kg) diminished NSAID- (and ethanol)-induced gastric lesions and DNA oxidation, restoring mitochondrial complexes activity, ARG1, GST-α protein levels and increasing HMOX-1 and SOD-2 expression. AP39 decreased proteins levels or phosphorylation of gastric mucosal inflammation/oxidation-sensitive markers and restored mTOR phosphorylation. Pharmacological inhibition of HMOX-1 attenuated AP39-gastroprotection. We showed that mitochondria-targeted H2S released from very low i.g. doses of AP39 improved gastric mucosal capacity to cope with NSAIDs-induced mitochondrial dysfunction and redox imbalance, mechanistically requiring the activity of HMOX-1.
Keywords: Gastric mucosa; Heme oxygenase; Hydrogen sulfide; Mitochondria; Non-steroidal anti-inflammatory drugs.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest These authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Matthew Whiteman reports a relationship with MitoRx Therapeutics that includes: equity or stocks. Roberta Torregrossa reports a relationship with MitoRx Therapeutics that includes: employment. Matthew Whiteman has patents awarded and pending for the use of sulfide-delivery molecules.
Figures


References
-
- Magierowski M., Magierowska K., Hubalewska-Mazgaj M., Sliwowski Z., Pajdo R., Ginter G., Kwiecien S., Brzozowski T., Nagahara N., Wrobel M. Exogenous and endogenous hydrogen sulfide protects gastric mucosa against the formation and time-dependent development of ischemia/reperfusion-induced acute lesions progressing into deeper ulcerations. Molecules. 2017;22(2) doi: 10.3390/molecules22020295. - DOI - PMC - PubMed
-
- Snijder P.M., Baratashvili M., Grzeschik N.A., Leuvenink H.G.D., Kuijpers L., Huitema S., Schaap O., Giepmans B.N.G., Kuipers J., Miljkovic J.L., Mitrovic A., Bos E.M., Szabó C., Kampinga H.H., Dijkers P.F., Den Dunnen W.F.A., Filipovic M.R., Van Goor H., Sibon O.C.M. Overexpression of cystathionine γ-lyase suppresses detrimental effects of spinocerebellar ataxia type 3. Mol. Med. 2015;21 doi: 10.2119/molmed.2015.00221. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

