Protective effects of luteolin against amyloid beta-induced oxidative stress and mitochondrial impairments through peroxisome proliferator-activated receptor γ-dependent mechanism in Alzheimer's disease
- PMID: 37597424
- PMCID: PMC10462892
- DOI: 10.1016/j.redox.2023.102848
Protective effects of luteolin against amyloid beta-induced oxidative stress and mitochondrial impairments through peroxisome proliferator-activated receptor γ-dependent mechanism in Alzheimer's disease
Abstract
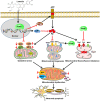
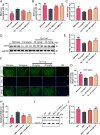
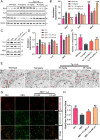
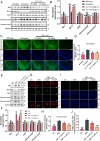
Alzheimer's disease (AD) is a devastating neurodegenerative disorder characterized by the deposition of β-amyloid (Aβ) peptides and dysfunction of mitochondrion, which result in neuronal apoptosis and ultimately cognitive impairment. Inhibiting Aβ generation and repairing mitochondrial damage are prominent strategies in AD therapeutic treatment. Luteolin, a flavonoid compound, exhibits anti-inflammatory neuroprotective properties in AD mice. However, it is still unclear whether luteolin has any effect on Aβ pathology and mitochondrial dysfunction. In this study, the beneficial effect and underlying mechanism of luteolin were investigated in triple transgenic AD (3 × Tg-AD) mice and primary neurons. Our study showed that luteolin supplement significantly ameliorated memory and cognitive impairment of AD mice and exerted neuroprotection by inhibiting Aβ generation, repairing mitochondrial damage and reducing neuronal apoptosis. Further research revealed that luteolin could directly bind with peroxisome proliferator-activated receptor gama (PPARγ) to promote its expression and function. In the culture of hippocampus-derived primary neurons, addition of PPARγ antagonist GW9662 or knockdown of PPARγ with its siRNA could eliminate the effect of luteolin on AD pathologies. In summary, this work revealed for the first time that luteolin effectively improved cognitive deficits of 3 × Tg-AD mice and inhibited Aβ-induced oxidative stress, mitochondrial dysfunction and neuronal apoptosis via PPARγ-dependent mechanism. Hence, luteolin has the potential to serve as a therapeutic agent against AD.
Keywords: Alzheimer's disease (AD); Apoptosis; Luteolin; Mitochondrial impairments; Peroxisome proliferator-activated receptor γ (PPARγ); β-amyloid (Aβ).
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no competing interests.
Figures








References
-
- 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17:327–406. - PubMed
-
- Klimova B., Maresova P., Kuca K. Non-pharmacological approaches to the prevention and treatment of Alzheimer's disease with respect to the rising treatment costs. Curr. Alzheimer Res. 2016;13:1249–1258. - PubMed
-
- Tanzi R.E., Moir R.D., Wagner S.L. Clearance of Alzheimer's Abeta peptide: the many roads to perdition. Neuron. 2004;43:605–608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

