A regulatory circuit controlled by extranuclear and nuclear retinoic acid receptor α determines T cell activation and function
- PMID: 37597518
- PMCID: PMC10552917
- DOI: 10.1016/j.immuni.2023.07.017
A regulatory circuit controlled by extranuclear and nuclear retinoic acid receptor α determines T cell activation and function
Abstract
Ligation of retinoic acid receptor alpha (RARα) by RA promotes varied transcriptional programs associated with immune activation and tolerance, but genetic deletion approaches suggest the impact of RARα on TCR signaling. Here, we examined whether RARα would exert roles beyond transcriptional regulation. Specific deletion of the nuclear isoform of RARα revealed an RARα isoform in the cytoplasm of T cells. Extranuclear RARα was rapidly phosphorylated upon TCR stimulation and recruited to the TCR signalosome. RA interfered with extranuclear RARα signaling, causing suboptimal TCR activation while enhancing FOXP3+ regulatory T cell conversion. TCR activation induced the expression of CRABP2, which translocates RA to the nucleus. Deletion of Crabp2 led to increased RA in the cytoplasm and interfered with signalosome-RARα, resulting in impaired anti-pathogen immunity and suppressed autoimmune disease. Our findings underscore the significance of subcellular RA/RARα signaling in T cells and identify extranuclear RARα as a component of the TCR signalosome and a determinant of immune responses.
Keywords: FOXP3(+) regulatory T cells; T cell receptor; ZAP-70; anti-pathogen immunity; autoimmune disease; cellular-retinoic-acid-binding protein; effector differentiation; extranuclear; nuclear receptor; proliferation; retinoic acid; retinoic acid receptor alpha; signal transduction.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





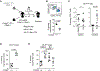
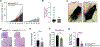
Comment in
-
cRARα: Derailleur nuclear retinoic acid chain to TCR.Allergy. 2024 May;79(5):1383-1385. doi: 10.1111/all.16034. Epub 2024 Jan 19. Allergy. 2024. PMID: 38240138 No abstract available.
References
-
- Larange A, Cheroutre H. Retinoic Acid and Retinoic Acid Receptors as Pleiotropic Modulators of the Immune System. Annu Rev Immunol. 2016;34:369–94. - PubMed
-
- di Masi A, Leboffe L, De Marinis E, Pagano F, Cicconi L, Rochette-Egly C, et al. Retinoic acid receptors: from molecular mechanisms to cancer therapy. Mol Aspects Med. 2015;41:1–115. - PubMed
-
- Farboud B, Privalsky ML. Retinoic acid receptor-alpha is stabilized in a repressive state by its C-terminal, isotype-specific F domain. Mol Endocrinol. 2004;18(12):2839–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

