Circulating tumor DNA association with residual cancer burden after neoadjuvant chemotherapy in triple-negative breast cancer in TBCRC 030
- PMID: 37597579
- PMCID: PMC10898256
- DOI: 10.1016/j.annonc.2023.08.004
Circulating tumor DNA association with residual cancer burden after neoadjuvant chemotherapy in triple-negative breast cancer in TBCRC 030
Abstract
Background: We aimed to examine circulating tumor DNA (ctDNA) and its association with residual cancer burden (RCB) using an ultrasensitive assay in patients with triple-negative breast cancer (TNBC) receiving neoadjuvant chemotherapy.
Patients and methods: We identified responders (RCB 0/1) and matched non-responders (RCB 2/3) from the phase II TBCRC 030 prospective study of neoadjuvant paclitaxel versus cisplatin in TNBC. We collected plasma samples at baseline, 3 weeks and 12 weeks (end of therapy). We created personalized ctDNA assays utilizing MAESTRO mutation enrichment sequencing. We explored associations between ctDNA and RCB status and disease recurrence.
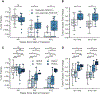
Results: Of 139 patients, 68 had complete samples and no additional neoadjuvant chemotherapy. Twenty-two were responders and 19 of those had sufficient tissue for whole-genome sequencing. We identified an additional 19 non-responders for a matched case-control analysis of 38 patients using a MAESTRO ctDNA assay tracking 319-1000 variants (median 1000 variants) to 114 plasma samples from 3 timepoints. Overall, ctDNA positivity was 100% at baseline, 79% at week 3 and 55% at week 12. Median tumor fraction (TFx) was 3.7 × 10-4 (range 7.9 × 10-7-4.9 × 10-1). TFx decreased 285-fold from baseline to week 3 in responders and 24-fold in non-responders. Week 12 ctDNA clearance correlated with RCB: clearance was observed in 10 of 11 patients with RCB 0, 3 of 8 with RCB 1, 4 of 15 with RCB 2 and 0 of 4 with RCB 3. Among six patients with known recurrence, five had persistent ctDNA at week 12.
Conclusions: Neoadjuvant chemotherapy for TNBC reduced ctDNA TFx by 285-fold in responders and 24-fold in non-responders. In 58% (22/38) of patients, ctDNA TFx dropped below the detection level of a commercially available test, emphasizing the need for sensitive tests. Additional studies will determine whether ctDNA-guided approaches can improve outcomes.
Keywords: biomarkers; circulating tumor DNA; minimal residual disease; recurrence; triple-negative breast cancer.
Copyright © 2023 European Society for Medical Oncology. Published by Elsevier Ltd. All rights reserved.
Figures

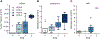

Update of
-
Circulating tumor DNA association with residual cancer burden after neoadjuvant chemotherapy in triple-negative breast cancer in TBCRC 030.medRxiv [Preprint]. 2023 Mar 8:2023.03.06.23286772. doi: 10.1101/2023.03.06.23286772. medRxiv. 2023. Update in: Ann Oncol. 2023 Oct;34(10):899-906. doi: 10.1016/j.annonc.2023.08.004. PMID: 36945501 Free PMC article. Updated. Preprint.
References
-
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010; 363 (20): 1938–1948. - PubMed
-
- Liedtke C, Mazouni C, Hess KR et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008; 26 (8): 1275–1281. - PubMed
-
- Dent R, Trudeau M, Pritchard KI et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007; 13 (15 Pt 1): 4429–4434. - PubMed
-
- Haffty BG, Yang Q, Reiss M et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol 2006; 24 (36): 5652–5657. - PubMed
-
- von Minckwitz G, Untch M, Blohmer JU et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012; 30 (15): 1796–1804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

